当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamic Modeling of Calcium Sulfate Hydrates in a CaSO4-H2SO4-H2O System from 273.15 to 473.15 K up to 5 m Sulfuric Acid.
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-03-30 , DOI: 10.1021/acs.jced.9b00829 Leiting Shen 1, 2 , Hannu Sippola 3, 4 , Xiaobin Li 2 , Daniel Lindberg 1 , Pekka Taskinen 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-03-30 , DOI: 10.1021/acs.jced.9b00829 Leiting Shen 1, 2 , Hannu Sippola 3, 4 , Xiaobin Li 2 , Daniel Lindberg 1 , Pekka Taskinen 1
Affiliation
|
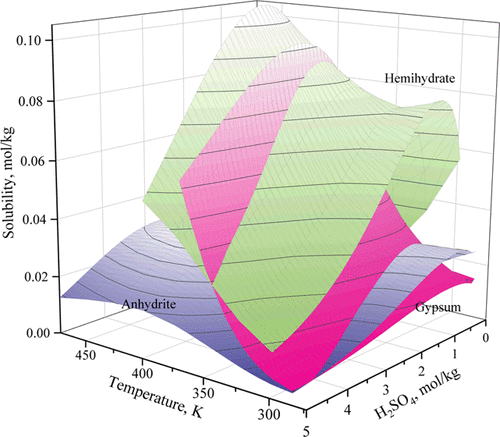
To prevent scaling and to recycle aqueous solutions in industrial processes, the thermodynamic properties of the CaSO4-H2SO4-H2O system are studied by thermodynamic modeling with the Pitzer model. The published solubility data of calcium sulfate hydrates in sulfuric acid solutions were collected and reviewed critically. Then, the CaSO4-H2SO4-H2O system was modeled using the Pitzer activity coefficient approach from critically selected experimental data to obtain optimized parameters. The model reproduces the solubility data with good accuracy up to 5 m sulfuric acid at temperatures of 283.15-368.15, 283.15-473.15, and 298.15-398.15 K for gypsum (CaSO4·2H2O), anhydrite (CaSO4), and hemihydrate (CaSO4·0.5H2O), respectively. However, at temperatures above 398.15 K and sulfuric acid concentration above 0.5 mol/kg, the solubility of anhydrite predicted by our model deviates significantly from the literature data. Our model predicts that the solubility of anhydrite would first increase but then decrease in more concentrated sulfuric acid solutions, which is in disagreement with the experimental data showing constantly increasing solubilities as a function of increasing sulfuric acid concentration. This discrepancy has been discussed. The transformations of gypsum to anhydrite and hemihydrate were predicted in sulfuric acid solutions. With increasing H2SO4 concentration, the transformation temperatures of gypsum to anhydrite and hemihydrate will decrease. Thus, gypsum is stable at low temperatures in solutions of low H2SO4 concentrations and transforms to anhydrite at high temperatures and in concentrated H2SO4 solutions, while hemihydrate is always a metastable phase. Furthermore, the predicted results were compared with previous experimental studies to verify the accuracy of the model.
中文翻译:

CaSO4-H2SO4-H2O系统中从273.15到473.15 K直至5 m硫酸的硫酸钙水合物的热力学建模。
为了防止结垢并回收工业过程中的水溶液,使用Pitzer模型通过热力学建模研究了CaSO4-H2SO4-H2O系统的热力学性质。收集并严格审查了公开发表的硫酸钙水合物在硫酸溶液中的溶解度数据。然后,使用Pitzer活性系数方法从严格选择的实验数据中对CaSO4-H2SO4-H2O系统进行建模,以获得优化的参数。该模型在石膏(CaSO4·2H2O),无水石膏(CaSO4)和半水合物(CaSO4·0.5)的温度为283.15-368.15、283.15-473.15和298.15-398.15 K的情况下,以高达5 m的硫酸准确地再现了溶解度数据。 H2O)。但是,在高于398.15 K的温度和高于0.5 mol / kg的硫酸浓度下,我们的模型预测的硬石膏的溶解度与文献数据有显着差异。我们的模型预测,在更浓的硫酸溶液中,硬石膏的溶解度会先增加然后降低,这与表明溶解度随着硫酸浓度的增加而不断增加的实验数据不一致。已经讨论了这种差异。预测在硫酸溶液中石膏向硬石膏和半水合物的转化。随着H2SO4浓度的增加,石膏向硬石膏和半水合物的转变温度将降低。因此,石膏在低浓度H2SO4的溶液中在低温下稳定,并在高温和浓H2SO4溶液中转变为硬石膏。而半水合物始终是亚稳相。此外,将预测结果与以前的实验研究进行了比较,以验证模型的准确性。
更新日期:2020-03-30
中文翻译:

CaSO4-H2SO4-H2O系统中从273.15到473.15 K直至5 m硫酸的硫酸钙水合物的热力学建模。
为了防止结垢并回收工业过程中的水溶液,使用Pitzer模型通过热力学建模研究了CaSO4-H2SO4-H2O系统的热力学性质。收集并严格审查了公开发表的硫酸钙水合物在硫酸溶液中的溶解度数据。然后,使用Pitzer活性系数方法从严格选择的实验数据中对CaSO4-H2SO4-H2O系统进行建模,以获得优化的参数。该模型在石膏(CaSO4·2H2O),无水石膏(CaSO4)和半水合物(CaSO4·0.5)的温度为283.15-368.15、283.15-473.15和298.15-398.15 K的情况下,以高达5 m的硫酸准确地再现了溶解度数据。 H2O)。但是,在高于398.15 K的温度和高于0.5 mol / kg的硫酸浓度下,我们的模型预测的硬石膏的溶解度与文献数据有显着差异。我们的模型预测,在更浓的硫酸溶液中,硬石膏的溶解度会先增加然后降低,这与表明溶解度随着硫酸浓度的增加而不断增加的实验数据不一致。已经讨论了这种差异。预测在硫酸溶液中石膏向硬石膏和半水合物的转化。随着H2SO4浓度的增加,石膏向硬石膏和半水合物的转变温度将降低。因此,石膏在低浓度H2SO4的溶液中在低温下稳定,并在高温和浓H2SO4溶液中转变为硬石膏。而半水合物始终是亚稳相。此外,将预测结果与以前的实验研究进行了比较,以验证模型的准确性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号