当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Multifunctional Nanocatalysts on n-C7 Asphaltene Adsorption and Subsequent Oxidation under High-Pressure Conditions
Energy & Fuels ( IF 5.2 ) Pub Date : 2020-03-29 , DOI: 10.1021/acs.energyfuels.0c00653 Oscar E. Medina 1 , Jaime Gallego 2 , Carol M. Olmos 1 , Xiaowei Chen 3 , Farid B. Cortés 1 , Camilo A. Franco 1
Energy & Fuels ( IF 5.2 ) Pub Date : 2020-03-29 , DOI: 10.1021/acs.energyfuels.0c00653 Oscar E. Medina 1 , Jaime Gallego 2 , Carol M. Olmos 1 , Xiaowei Chen 3 , Farid B. Cortés 1 , Camilo A. Franco 1
Affiliation
|
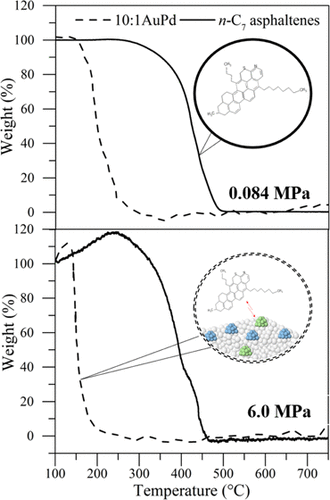
This study was carried out to evaluate the effect of high pressure on the oxidation kinetics of n-heptane asphaltenes in the presence and absence of AuPd/Ce0.62Zr0.38O2 catalysts. Bimetallic Au–Pd catalysts with Au/Pd molar ratios from 3.5 to 9.6 were synthesized by deposition–precipitation of Au and followed by incipient wetness impregnation of Pd (3:1AuPd and 10:1AuPd). Adsorption isotherms between hydrocarbons and nanocatalysts were constructed varying the initial asphaltene concentration from 100 to 1500 mg·L–1. Subsequent oxidation was evaluated using thermogravimetric analysis under an air atmosphere, at different pressures from 0.084 to 6.0 MPa in a wide temperature range between 100 and 800 °C. Kinetic parameters were calculated using a first-order kinetic model, considering the system pressure. Adsorption affinity increases in the order support < 3:1AuPd < 10:1AuPd. The catalytic activity of the nanocatalyst was highly dependent on the employed temperature and pressure. Their presence reduces the n-C7 asphaltene decomposition temperature from 450 °C to temperatures below 200 °C for all catalysts used at 6.0 MPa. The main decomposition peaks are presented at 150, 170, and 210 °C for 10:1AuPd, 3:1AuPd, and support, at 6.0 MPa, respectively. Besides, the oxygen chemisorption (OC) region is favored as the material has a greater catalytic activity, increasing from 9.0 to 14.0% (on the load asphaltene basis calculation) for the support and 10:1AuPd catalyst. This was corroborated by the activation energy, which is reduced by more than 30% for all pressures evaluated with the best system. Besides, 89.0 and 80.0% of the mass are lost in the decomposition of the chemisorbed oxygen (DCO) thermal event for the 10:1AuPd and 3:1AuPd nanocatalysts, respectively. Finally, first and second combustions were carried out at temperatures below 240 °C at 6.0 MPa for the 10:1AuPd system, which is a very promising result to determine the reaction pathway for the heavy and extraheavy crude oils during EOR application.
中文翻译:

多功能纳米催化剂对高压条件下n -C 7沥青吸附及后续氧化的影响
进行该研究以评估在有和没有AuPd / Ce 0.62 Zr 0.38 O 2催化剂的情况下高压对正庚烷沥青烯氧化动力学的影响。通过沉积-沉淀Au,然后初湿浸渍Pd(3:1AuPd和10:1AuPd),合成了Au / Pd摩尔比为3.5至9.6的双金属Au-Pd催化剂。构建了碳氢化合物和纳米催化剂之间的吸附等温线,其初始沥青质浓度从100到1500 mg·L –1。在空气气氛中,在100至800°C的宽温度范围内,在0.084至6.0 MPa的不同压力下,使用热重分析法评估随后的氧化。考虑到系统压力,使用一阶动力学模型计算动力学参数。吸附亲和力以支持度<3:1AuPd <10:1AuPd的顺序增加。纳米催化剂的催化活性高度取决于所采用的温度和压力。它们的存在会降低n -C 7对于在6.0 MPa下使用的所有催化剂,沥青质的分解温度从450°C到低于200°C。对于10:1AuPd,3:1AuPd和载体,在6.0 MPa时,主要分解峰分别出现在150、170和210°C下。此外,氧化学吸附(OC)区域是有利的,因为该材料具有更大的催化活性,对于载体和10:1AuPd催化剂,其从9.0%增加到14.0%(基于负载沥青质计算)。活化能证实了这一点,对于采用最佳系统评估的所有压力,活化能均降低了30%以上。此外,在10:1AuPd和3:1AuPd纳米催化剂的化学吸附氧(DCO)热事件的分解中,分别损失了89.0%和80.0%的质量。最后,第一次燃烧和第二次燃烧是在低于240°C的温度下于6进行的。
更新日期:2020-03-29
中文翻译:

多功能纳米催化剂对高压条件下n -C 7沥青吸附及后续氧化的影响
进行该研究以评估在有和没有AuPd / Ce 0.62 Zr 0.38 O 2催化剂的情况下高压对正庚烷沥青烯氧化动力学的影响。通过沉积-沉淀Au,然后初湿浸渍Pd(3:1AuPd和10:1AuPd),合成了Au / Pd摩尔比为3.5至9.6的双金属Au-Pd催化剂。构建了碳氢化合物和纳米催化剂之间的吸附等温线,其初始沥青质浓度从100到1500 mg·L –1。在空气气氛中,在100至800°C的宽温度范围内,在0.084至6.0 MPa的不同压力下,使用热重分析法评估随后的氧化。考虑到系统压力,使用一阶动力学模型计算动力学参数。吸附亲和力以支持度<3:1AuPd <10:1AuPd的顺序增加。纳米催化剂的催化活性高度取决于所采用的温度和压力。它们的存在会降低n -C 7对于在6.0 MPa下使用的所有催化剂,沥青质的分解温度从450°C到低于200°C。对于10:1AuPd,3:1AuPd和载体,在6.0 MPa时,主要分解峰分别出现在150、170和210°C下。此外,氧化学吸附(OC)区域是有利的,因为该材料具有更大的催化活性,对于载体和10:1AuPd催化剂,其从9.0%增加到14.0%(基于负载沥青质计算)。活化能证实了这一点,对于采用最佳系统评估的所有压力,活化能均降低了30%以上。此外,在10:1AuPd和3:1AuPd纳米催化剂的化学吸附氧(DCO)热事件的分解中,分别损失了89.0%和80.0%的质量。最后,第一次燃烧和第二次燃烧是在低于240°C的温度下于6进行的。









































 京公网安备 11010802027423号
京公网安备 11010802027423号