当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Can Isoprene Oxidation Explain High Concentrations of Atmospheric Formic and Acetic Acid over Forests?
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-03-30 , DOI: 10.1021/acsearthspacechem.0c00010 Michael F. Link 1 , Tran B. Nguyen 2 , Kelvin Bates 3 , Jean-François Müller 4 , Delphine K. Farmer 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-03-30 , DOI: 10.1021/acsearthspacechem.0c00010 Michael F. Link 1 , Tran B. Nguyen 2 , Kelvin Bates 3 , Jean-François Müller 4 , Delphine K. Farmer 1
Affiliation
|
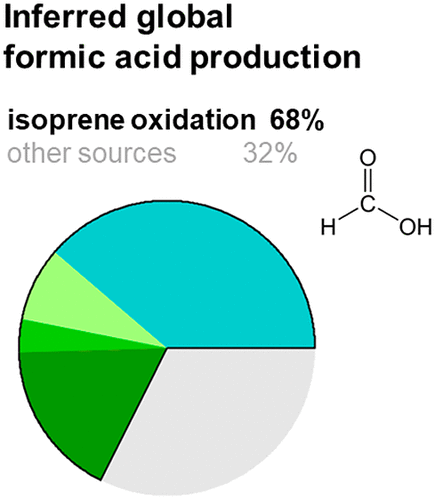
Formic and acetic acid concentrations are particularly high over forested areas of the world. However, the gas-phase mechanisms for producing these acids are poorly understood even for isoprene, the globally dominant biogenic hydrocarbon. We quantified formic and acetic acid production from reactions of hydroxyl radical (OH) (between high and low ranges of nitric oxide (NO) levels) with isoprene, methacrolein (MACR), isoprene epoxydiol (IEPOX), isoprene hydroxy hydroperoxide (ISOPOOH), and α-pinene from the focused isoprene experiments at California Institute of Technology (FIXCIT) laboratory chamber study. We find that (i) OH oxidation of MACR, IEPOX, and ISOPOOH are sources of formic acid, (ii) isoprene peroxy radical isomerization and associated photolysis oxidation products are potentially important sources of organic acids, and (iii) high levels of NO generally suppress organic acid formation from OH oxidation of isoprene. We modified existing chemical mechanisms for isoprene oxidation to account for organic acid production pathways observed in the FIXCIT study. We simulated organic acid production during the Southeastern Oxidant and Aerosol Study using the updated chemical mechanisms and represented acetic acid within a factor of 2 but still underpredicted formic acid by a factor of 6. While we cannot explain ambient formic acid with explicit chemical mechanisms, the FIXCIT results suggest that the oxidation of isoprene could account for as much as 70% of the global annual production of formic acid from gas-phase reactions.
中文翻译:

异戊二烯氧化能解释森林中高浓度的大气甲酸和乙酸吗?
在世界上森林茂密的地区,甲酸和乙酸的浓度特别高。但是,即使对于异戊二烯(全球占主导地位的生物烃),生产这些酸的气相机理也知之甚少。我们量化了羟基自由基(OH)(在一氧化氮(NO)水平的高低范围之间)与异戊二烯,甲基丙烯醛(MACR),异戊二烯环氧二醇(IEPOX),异戊二烯羟基氢过氧化物(ISOPOOH),和α-pine烯来自加利福尼亚理工学院(FIXCIT)实验室研究的重点异戊二烯实验。我们发现(i)MACR,IEPOX和ISOPOOH的OH氧化是甲酸的来源,(ii)异戊二烯过氧自由基异构化和相关的光解氧化产物是有机酸的潜在重要来源,(iii)高水平的NO通常会抑制异戊二烯的OH氧化形成有机酸。我们修改了现有的异戊二烯氧化化学机制,以解决FIXCIT研究中观察到的有机酸产生途径。我们使用最新的化学机理模拟了东南氧化剂和气溶胶研究期间的有机酸生产,并将乙酸表示为2的因数,但仍将甲酸的预测不足为6。虽然我们无法通过明确的化学机理来解释环境甲酸,但FIXCIT结果表明,异戊二烯的氧化可占气相反应甲酸全球年产量的70%。我们修改了现有的异戊二烯氧化化学机制,以解决FIXCIT研究中观察到的有机酸产生途径。我们使用最新的化学机理模拟了东南氧化剂和气溶胶研究期间的有机酸生产,并将乙酸表示为2的因数,但仍将甲酸的预测不足为6。虽然我们无法通过明确的化学机理来解释环境甲酸,但FIXCIT结果表明,异戊二烯的氧化可占气相反应甲酸全球年产量的70%。我们修改了现有的异戊二烯氧化化学机制,以解决FIXCIT研究中观察到的有机酸产生途径。我们使用最新的化学机理模拟了东南氧化剂和气溶胶研究期间的有机酸生产,并将乙酸表示为2的因数,但仍将甲酸的预测不足为6。虽然我们无法通过明确的化学机理来解释环境甲酸,但FIXCIT结果表明,异戊二烯的氧化可占气相反应甲酸全球年产量的70%。
更新日期:2020-03-30
中文翻译:

异戊二烯氧化能解释森林中高浓度的大气甲酸和乙酸吗?
在世界上森林茂密的地区,甲酸和乙酸的浓度特别高。但是,即使对于异戊二烯(全球占主导地位的生物烃),生产这些酸的气相机理也知之甚少。我们量化了羟基自由基(OH)(在一氧化氮(NO)水平的高低范围之间)与异戊二烯,甲基丙烯醛(MACR),异戊二烯环氧二醇(IEPOX),异戊二烯羟基氢过氧化物(ISOPOOH),和α-pine烯来自加利福尼亚理工学院(FIXCIT)实验室研究的重点异戊二烯实验。我们发现(i)MACR,IEPOX和ISOPOOH的OH氧化是甲酸的来源,(ii)异戊二烯过氧自由基异构化和相关的光解氧化产物是有机酸的潜在重要来源,(iii)高水平的NO通常会抑制异戊二烯的OH氧化形成有机酸。我们修改了现有的异戊二烯氧化化学机制,以解决FIXCIT研究中观察到的有机酸产生途径。我们使用最新的化学机理模拟了东南氧化剂和气溶胶研究期间的有机酸生产,并将乙酸表示为2的因数,但仍将甲酸的预测不足为6。虽然我们无法通过明确的化学机理来解释环境甲酸,但FIXCIT结果表明,异戊二烯的氧化可占气相反应甲酸全球年产量的70%。我们修改了现有的异戊二烯氧化化学机制,以解决FIXCIT研究中观察到的有机酸产生途径。我们使用最新的化学机理模拟了东南氧化剂和气溶胶研究期间的有机酸生产,并将乙酸表示为2的因数,但仍将甲酸的预测不足为6。虽然我们无法通过明确的化学机理来解释环境甲酸,但FIXCIT结果表明,异戊二烯的氧化可占气相反应甲酸全球年产量的70%。我们修改了现有的异戊二烯氧化化学机制,以解决FIXCIT研究中观察到的有机酸产生途径。我们使用最新的化学机理模拟了东南氧化剂和气溶胶研究期间的有机酸生产,并将乙酸表示为2的因数,但仍将甲酸的预测不足为6。虽然我们无法通过明确的化学机理来解释环境甲酸,但FIXCIT结果表明,异戊二烯的氧化可占气相反应甲酸全球年产量的70%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号