当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dearomatization-Rearomatization Strategy for Synthesizing Carbazoles with 2,2'-Biphenols and Ammonia by Dual C(Ar)-OH Bond Cleavages.
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-03-30 , DOI: 10.1021/acs.jafc.0c00644 Dawei Cao 1 , Jing Yu 1 , Huiying Zeng 1 , Chao-Jun Li 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-03-30 , DOI: 10.1021/acs.jafc.0c00644 Dawei Cao 1 , Jing Yu 1 , Huiying Zeng 1 , Chao-Jun Li 2
Affiliation
|
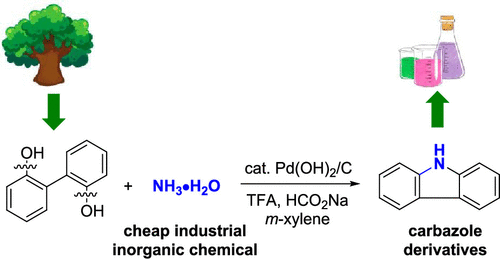
Carbazole is an essential building block in various pharmaceuticals, agrochemicals, natural products, and materials. For future sustainability, it is highly desirable to synthesize carbazole derivatives directly from renewable resources or cheap raw materials. Phenolic compounds are a class of degradation products of lignin. On the other hand, ammonia is a very cheap industrial inorganic chemical. Herein, an efficient dearomatization–rearomatization strategy has been developed to directly cross-couple 2,2′-biphenols with ammonia by dual C(Ar)–OH bond cleavages. This strategy provides a greener pathway to synthesize valuable carbazole derivatives from phenols.
中文翻译:

通过双C(Ar)-OH键裂解合成具有2,2'-联苯酚和氨的咔唑的脱芳香化-再芳香化策略。
咔唑是各种药物,农用化学品,天然产物和材料中必不可少的组成部分。为了将来的可持续性,非常需要直接从可再生资源或廉价原料中合成咔唑衍生物。酚类化合物是木质素的一类降解产物。另一方面,氨是一种非常便宜的工业无机化学品。在这里,已经开发了一种有效的脱芳香化-再芳香化策略,可以通过双C(Ar)-OH键裂解直接将2,2'-双酚与氨进行交叉偶联。该策略为从酚类合成有价值的咔唑衍生物提供了更绿色的途径。
更新日期:2020-03-30
中文翻译:

通过双C(Ar)-OH键裂解合成具有2,2'-联苯酚和氨的咔唑的脱芳香化-再芳香化策略。
咔唑是各种药物,农用化学品,天然产物和材料中必不可少的组成部分。为了将来的可持续性,非常需要直接从可再生资源或廉价原料中合成咔唑衍生物。酚类化合物是木质素的一类降解产物。另一方面,氨是一种非常便宜的工业无机化学品。在这里,已经开发了一种有效的脱芳香化-再芳香化策略,可以通过双C(Ar)-OH键裂解直接将2,2'-双酚与氨进行交叉偶联。该策略为从酚类合成有价值的咔唑衍生物提供了更绿色的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号