当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Boron–Boron Double Transborylation Strategy for the Synthesis of gem-Diborylalkanes
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-03-30 , DOI: 10.1021/acscatal.0c00869 Jamie H. Docherty 1 , Kieran Nicholson 1 , Andrew P. Dominey 2 , Stephen P. Thomas 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-03-30 , DOI: 10.1021/acscatal.0c00869 Jamie H. Docherty 1 , Kieran Nicholson 1 , Andrew P. Dominey 2 , Stephen P. Thomas 1
Affiliation
|
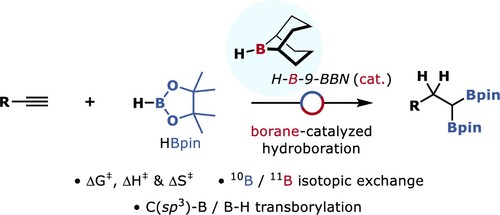
Olefin hydroboration reactions provide efficient access to synthetically versatile and easily handled organoboronic esters. In this study, we demonstrate that the commercially available organoborane reagent 9-borabicyclo[3.3.1]nonane (H-B-9-BBN) can serve as a catalyst for the sequential double hydroboration of alkynes using pinacolborane (HBpin). This strategy, which is effective for a wide range of terminal alkynes, is predicated upon a key C(sp3)−B/B–H transborylation reaction. Transition-state thermodynamic parameters and 10-boron-isotopic labeling experiments are indicative of a σ-bond metathesis exchange pathway.
中文翻译:

硼-硼双转硼烷基化策略合成宝石-二硼烷基烷烃
烯烃加氢硼化反应可有效地获得合成用途广泛且易于处理的有机硼酸酯。在这项研究中,我们表明,商购的有机基硼烷试剂9-硼杂双环[3.3.1]壬烷(H-乙-9-BBN)可以作为使用频哪醇(HBpin)炔烃组成的连续双硼氢化的催化剂。此策略对广泛的末端炔烃有效,基于关键的C(sp 3)-B / B-H跨碱化反应。过渡态热力学参数和10硼同位素标记实验表明σ键易位交换途径。
更新日期:2020-04-23
中文翻译:

硼-硼双转硼烷基化策略合成宝石-二硼烷基烷烃
烯烃加氢硼化反应可有效地获得合成用途广泛且易于处理的有机硼酸酯。在这项研究中,我们表明,商购的有机基硼烷试剂9-硼杂双环[3.3.1]壬烷(H-乙-9-BBN)可以作为使用频哪醇(HBpin)炔烃组成的连续双硼氢化的催化剂。此策略对广泛的末端炔烃有效,基于关键的C(sp 3)-B / B-H跨碱化反应。过渡态热力学参数和10硼同位素标记实验表明σ键易位交换途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号