当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering cytochrome P450s for enantioselective cyclopropenation of internal alkynes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-29 , DOI: 10.1021/jacs.0c01313 Kai Chen 1 , Frances H. Arnold 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-29 , DOI: 10.1021/jacs.0c01313 Kai Chen 1 , Frances H. Arnold 1
Affiliation
|
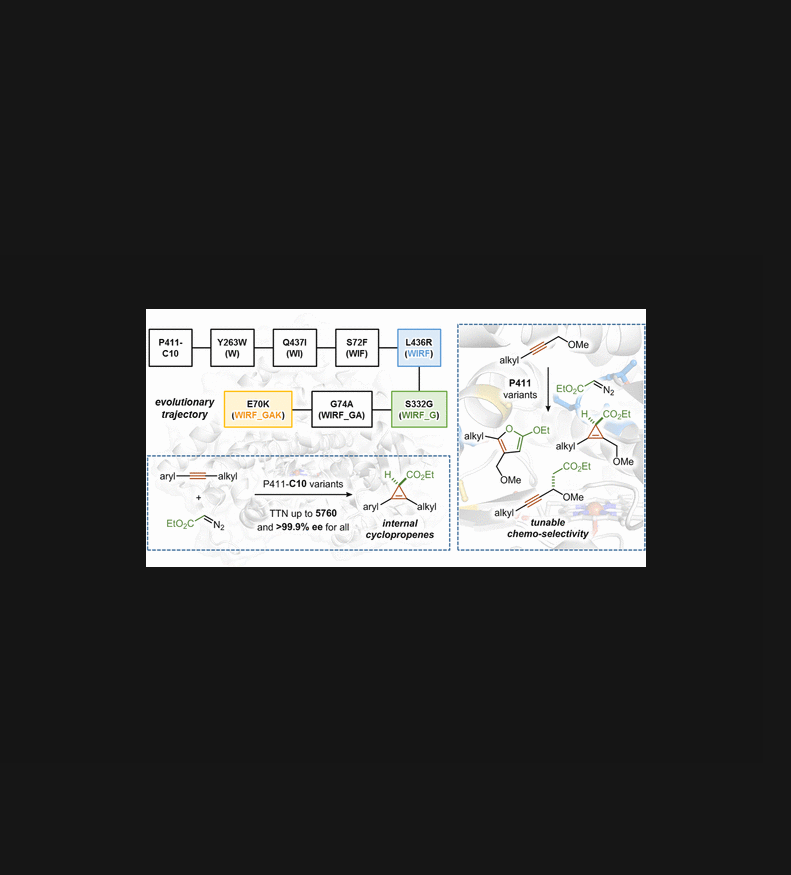
We report a biocatalytic platform of engineered cytochrome P450 enzymes to carry out efficient cyclopropene synthesis via carbene transfer to internal alkynes. Directed evolution of a serine-ligated P450 variant, P411-C10, yielded a lineage of engineered P411 enzymes that together accommodate a variety of internal aromatic alkynes as substrates for cyclopropenation with unprecedented efficiencies and stereoselectivities (up to 5760 TTN, and all with >99.9% ee). Using an internal aliphatic alkyne bearing a propargylic ether group, different P411 variants can selectively catalyze cyclopropene formation, carbene insertion into a propargylic C‒H bond or [3+2]-cycloaddition. This tunable reaction selectivity further highlights the benefit of using genetically-encoded catalysts to address chemoselectivity challenges.
中文翻译:

工程细胞色素 P450s 用于内部炔烃的对映选择性环丙烯化
我们报告了一种工程细胞色素 P450 酶的生物催化平台,通过将卡宾转移到内部炔烃来进行有效的环丙烯合成。丝氨酸连接的 P450 变体 P411-C10 的定向进化产生了一系列工程化的 P411 酶,这些酶共同容纳各种内部芳香炔作为环丙烯化的底物,具有前所未有的效率和立体选择性(高达 5760 TTN,并且均具有 >99.9 % ee)。使用带有炔丙基醚基团的内部脂肪族炔烃,不同的 P411 变体可以选择性地催化环丙烯形成、卡宾插入炔丙基 C-H 键或 [3+2]-环加成。这种可调节的反应选择性进一步突出了使用基因编码催化剂解决化学选择性挑战的好处。
更新日期:2020-03-29
中文翻译:

工程细胞色素 P450s 用于内部炔烃的对映选择性环丙烯化
我们报告了一种工程细胞色素 P450 酶的生物催化平台,通过将卡宾转移到内部炔烃来进行有效的环丙烯合成。丝氨酸连接的 P450 变体 P411-C10 的定向进化产生了一系列工程化的 P411 酶,这些酶共同容纳各种内部芳香炔作为环丙烯化的底物,具有前所未有的效率和立体选择性(高达 5760 TTN,并且均具有 >99.9 % ee)。使用带有炔丙基醚基团的内部脂肪族炔烃,不同的 P411 变体可以选择性地催化环丙烯形成、卡宾插入炔丙基 C-H 键或 [3+2]-环加成。这种可调节的反应选择性进一步突出了使用基因编码催化剂解决化学选择性挑战的好处。











































 京公网安备 11010802027423号
京公网安备 11010802027423号