当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Tail-to-Head Cyclizations Catalyzed by Dual-Hydrogen-Bond Donors
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-28 , DOI: 10.1021/jacs.0c02665 Dennis A Kutateladze 1 , Daniel A Strassfeld 1 , Eric N Jacobsen 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-28 , DOI: 10.1021/jacs.0c02665 Dennis A Kutateladze 1 , Daniel A Strassfeld 1 , Eric N Jacobsen 1
Affiliation
|
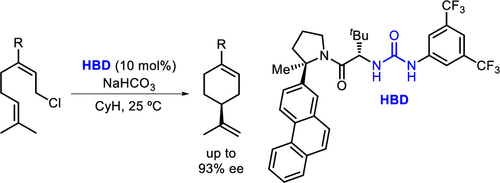
Chiral urea derivatives are shown to catalyze enantioselective tail-to-head cyclization reactions of neryl chloride analogues. Experimental data are consistent with a mechanism in which π-participation by the nucleophilic olefin facilitates chloride ionization and thereby circumvents simple elimination pathways. Kinetic and computational studies support a cooperative mode of catalysis wherein two molecules of the urea catalyst engage the substrate and induce enantioselectivity through selective transition state stabilization.
中文翻译:

双氢键供体催化的对映选择性尾对头环化
手性脲衍生物可催化橙花基氯类似物的对映选择性尾对头环化反应。实验数据与亲核烯烃的π参与促进氯离子化并从而绕过简单的消除途径的机制一致。动力学和计算研究支持协同催化模式,其中尿素催化剂的两个分子与底物结合并通过选择性过渡态稳定诱导对映选择性。
更新日期:2020-03-28
中文翻译:

双氢键供体催化的对映选择性尾对头环化
手性脲衍生物可催化橙花基氯类似物的对映选择性尾对头环化反应。实验数据与亲核烯烃的π参与促进氯离子化并从而绕过简单的消除途径的机制一致。动力学和计算研究支持协同催化模式,其中尿素催化剂的两个分子与底物结合并通过选择性过渡态稳定诱导对映选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号