当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
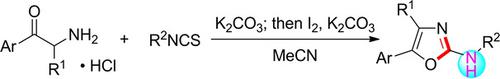
Synthesis of 2‐Amino Substituted Oxazoles from α‐Amino Ketones and Isothiocyanates via Sequential Addition and I2‐Mediated Desulfurative Cyclization
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-03-27 , DOI: 10.1002/adsc.202000171 Shuangshuang Zhang 1 , Qiongli Zhao 1 , Yifei Zhao 1 , Wenquan Yu 1 , Junbiao Chang 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-03-27 , DOI: 10.1002/adsc.202000171 Shuangshuang Zhang 1 , Qiongli Zhao 1 , Yifei Zhao 1 , Wenquan Yu 1 , Junbiao Chang 1
Affiliation
|
Oxazol‐2‐amines were synthesized by annulation of α‐amino ketones and isothiocyanates. This sequential synthetic process involves addition of α‐amino ketones to isothiocyanates and I2‐promoted desulfurative cyclization omitting isolation of the less stable thiourea intermediates. It is transition metal‐free and operationally simple, providing access to a variety of 2‐amino substituted oxazole derivatives under mild reaction conditions.
中文翻译:

通过顺序加成和I2介导的脱硫环化反应,由α-氨基酮和异硫氰酸酯合成2-氨基取代的恶唑
草酸-2-胺是通过环α-氨基酮和异硫氰酸酯合成的。该顺序合成过程涉及将α-氨基酮添加至异硫氰酸酯和I 2促进的脱硫环化反应,从而省去了较不稳定的硫脲中间体的分离。它不含过渡金属,操作简单,可在温和的反应条件下使用各种2-氨基取代的恶唑衍生物。
更新日期:2020-03-27
中文翻译:

通过顺序加成和I2介导的脱硫环化反应,由α-氨基酮和异硫氰酸酯合成2-氨基取代的恶唑
草酸-2-胺是通过环α-氨基酮和异硫氰酸酯合成的。该顺序合成过程涉及将α-氨基酮添加至异硫氰酸酯和I 2促进的脱硫环化反应,从而省去了较不稳定的硫脲中间体的分离。它不含过渡金属,操作简单,可在温和的反应条件下使用各种2-氨基取代的恶唑衍生物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号