Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients With Heart Failure With and Without Diabetes
JAMA ( IF 63.1 ) Pub Date : 2020-04-14 , DOI: 10.1001/jama.2020.1906 Mark C. Petrie, Subodh Verma, Kieran F. Docherty, Silvio E. Inzucchi, Inder Anand, Jan Belohlávek, Michael Böhm, Chern-En Chiang, Vijay K. Chopra, Rudolf A. de Boer, Akshay S. Desai, Mirta Diez, Jaroslaw Drozdz, Andre Dukát, Junbo Ge, Jonathan Howlett, Tzvetana Katova, Masafumi Kitakaze, Charlotta E. A. Ljungman, Béla Merkely, Jose C. Nicolau, Eileen O'Meara, Pham Nguyen Vinh, Morten Schou, Sergey Tereshchenko, Lars Køber, Mikhail N. Kosiborod, Anna Maria Langkilde, Felipe A. Martinez, Piotr Ponikowski, Marc S. Sabatine, Mikaela Sjöstrand, Scott D. Solomon, Per Johanson, Peter J. Greasley, David Boulton, Olof Bengtsson, Pardeep S. Jhund, John J. V. McMurray
JAMA ( IF 63.1 ) Pub Date : 2020-04-14 , DOI: 10.1001/jama.2020.1906 Mark C. Petrie, Subodh Verma, Kieran F. Docherty, Silvio E. Inzucchi, Inder Anand, Jan Belohlávek, Michael Böhm, Chern-En Chiang, Vijay K. Chopra, Rudolf A. de Boer, Akshay S. Desai, Mirta Diez, Jaroslaw Drozdz, Andre Dukát, Junbo Ge, Jonathan Howlett, Tzvetana Katova, Masafumi Kitakaze, Charlotta E. A. Ljungman, Béla Merkely, Jose C. Nicolau, Eileen O'Meara, Pham Nguyen Vinh, Morten Schou, Sergey Tereshchenko, Lars Køber, Mikhail N. Kosiborod, Anna Maria Langkilde, Felipe A. Martinez, Piotr Ponikowski, Marc S. Sabatine, Mikaela Sjöstrand, Scott D. Solomon, Per Johanson, Peter J. Greasley, David Boulton, Olof Bengtsson, Pardeep S. Jhund, John J. V. McMurray
|
Importance
Additional treatments are needed for heart failure with reduced ejection fraction (HFrEF). Sodium-glucose cotransporter 2 (SGLT2) inhibitors may be an effective treatment for patients with HFrEF, even those without diabetes. Objective
To evaluate the effects of dapagliflozin in patients with HFrEF with and without diabetes. Design, Setting, and Participants
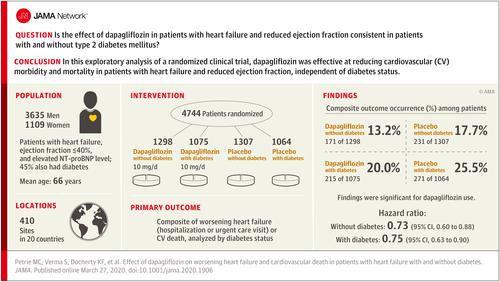
Exploratory analysis of a phase 3 randomized trial conducted at 410 sites in 20 countries. Patients with New York Heart Association classification II to IV with an ejection fraction less than or equal to 40% and elevated plasma N-terminal pro B-type natriuretic peptide were enrolled between February 15, 2017, and August 17, 2018, with final follow-up on June 6, 2019. Interventions
Addition of once-daily 10 mg of dapagliflozin or placebo to recommended therapy. Main Outcomes and Measures
The primary outcome was the composite of an episode of worsening heart failure or cardiovascular death. This outcome was analyzed by baseline diabetes status and, in patients without diabetes, by glycated hemoglobin level less than 5.7% vs greater than or equal to 5.7%. Results
Among 4744 patients randomized (mean age, 66 years; 1109 [23%] women; 2605 [55%] without diabetes), 4742 completed the trial. Among participants without diabetes, the primary outcome occurred in 171 of 1298 (13.2%) in the dapagliflozin group and 231 of 1307 (17.7%) in the placebo group (hazard ratio, 0.73 [95% CI, 0.60-0.88]). In patients with diabetes, the primary outcome occurred in 215 of 1075 (20.0%) in the dapagliflozin group and 271 of 1064 (25.5%) in the placebo group (hazard ratio, 0.75 [95% CI, 0.63-0.90]) (P value for interaction = .80). Among patients without diabetes and a glycated hemoglobin level less than 5.7%, the primary outcome occurred in 53 of 438 patients (12.1%) in the dapagliflozin group and 71 of 419 (16.9%) in the placebo group (hazard ratio, 0.67 [95% CI, 0.47-0.96]). In patients with a glycated hemoglobin of at least 5.7%, the primary outcome occurred in 118 of 860 patients (13.7%) in the dapagliflozin group and 160 of 888 (18.0%) in the placebo group (hazard ratio, 0.74 [95% CI, 0.59-0.94]) (P value for interaction = .72). Volume depletion was reported as an adverse event in 7.3% of patients in the dapagliflozin group and 6.1% in the placebo group among patients without diabetes and in 7.8% of patients in the dapagliflozin group and 7.8% in the placebo group among patients with diabetes. A kidney adverse event was reported in 4.8% of patients in the dapagliflozin group and 6.0% in the placebo group among patients without diabetes and in 8.5% of patients in the dapagliflozin group and 8.7% in the placebo group among patients with diabetes. Conclusions and Relevance
In this exploratory analysis of a randomized trial of patients with HFrEF, dapagliflozin compared with placebo, when added to recommended therapy, significantly reduced the risk of worsening heart failure or cardiovascular death independently of diabetes status. Trial Registration
ClinicalTrials.gov Identifier: NCT03036124.
中文翻译:

达格列净对合并和不合并糖尿病心力衰竭患者心力衰竭恶化和心血管死亡的影响
重要性 射血分数降低的心力衰竭 (HFrEF) 需要额外的治疗。钠-葡萄糖协同转运蛋白 2 (SGLT2) 抑制剂可能是 HFrEF 患者的有效治疗方法,即使是那些没有糖尿病的患者。目的评价达格列净对合并和不合并糖尿病的 HFrEF 患者的影响。设计、设置和参与者 在 20 个国家的 410 个地点进行的 3 期随机试验的探索性分析。2017 年 2 月 15 日至 2018 年 8 月 17 日期间,纳入纽约心脏协会 II 至 IV 级射血分数小于或等于 40% 且血浆 N 端 B 型利钠肽前体升高的患者,并进行最终随访- 2019 年 6 月 6 日更新。 干预 在推荐疗法中加入每日一次 10 毫克的达格列净或安慰剂。主要结果和测量主要结果是心力衰竭恶化或心血管死亡事件的复合。这一结果通过基线糖尿病状态进行分析,在没有糖尿病的患者中,通过糖化血红蛋白水平低于 5.7% 与大于或等于 5.7% 进行分析。结果 在随机分配的 4744 名患者中(平均年龄 66 岁;1109 名 [23%] 女性;2605 名 [55%] 没有糖尿病),4742 名完成了试验。在没有糖尿病的参与者中,主要结局发生在达格列净组 1298 名中的 171 名 (13.2%) 和安慰剂组 1307 名中的 231 名 (17.7%)(风险比,0.73 [95% CI,0.60-0.88])。在糖尿病患者中,达格列净组 1075 名患者中的 215 名 (20.0%) 和安慰剂组 1064 名患者中的 271 名 (25.5%) 发生主要结局(风险比,0.75 [95% CI,0.63-0.90])(P交互值 = .80)。在无糖尿病且糖化血红蛋白水平低于 5.7% 的患者中,主要结局发生在达格列净组 438 名患者中的 53 名 (12.1%) 和安慰剂组 419 名患者中的 71 名 (16.9%)(风险比,0.67 [95 % CI,0.47-0.96])。在糖化血红蛋白至少为 5.7% 的患者中,主要结局发生在达格列净组 860 名患者中的 118 名 (13.7%) 和安慰剂组 888 名患者中的 160 名 (18.0%)(风险比,0.74 [95% CI] , 0.59-0.94])(交互作用的 P 值 = .72)。无糖尿病患者中,达格列净组 7.3% 的患者和安慰剂组 6.1% 的患者以及糖尿病患者中 7.8% 的达格列净组患者和 7.8% 的安慰剂组患者将容量不足报告为不良事件。4 例报告了肾脏不良事件。在未患糖尿病的患者中,达格列净组中 8% 的患者和安慰剂组中 6.0% 的患者,以及糖尿病患者中达格列净组中 8.5% 的患者和安慰剂组中 8.7% 的患者。结论和相关性 在这项针对 HFrEF 患者的随机试验的探索性分析中,与安慰剂相比,当将达格列净添加到推荐治疗中时,可显着降低心力衰竭恶化或心血管死亡的风险,而与糖尿病状态无关。试验注册 ClinicalTrials.gov 标识符:NCT03036124。与安慰剂相比,dapagliflozin 加入推荐治疗后,可显着降低心力衰竭恶化或心血管死亡的风险,而与糖尿病状态无关。试验注册 ClinicalTrials.gov 标识符:NCT03036124。与安慰剂相比,dapagliflozin 添加到推荐治疗中后,可显着降低心力衰竭恶化或心血管死亡的风险,而与糖尿病状态无关。试验注册 ClinicalTrials.gov 标识符:NCT03036124。
更新日期:2020-04-14
中文翻译:

达格列净对合并和不合并糖尿病心力衰竭患者心力衰竭恶化和心血管死亡的影响
重要性 射血分数降低的心力衰竭 (HFrEF) 需要额外的治疗。钠-葡萄糖协同转运蛋白 2 (SGLT2) 抑制剂可能是 HFrEF 患者的有效治疗方法,即使是那些没有糖尿病的患者。目的评价达格列净对合并和不合并糖尿病的 HFrEF 患者的影响。设计、设置和参与者 在 20 个国家的 410 个地点进行的 3 期随机试验的探索性分析。2017 年 2 月 15 日至 2018 年 8 月 17 日期间,纳入纽约心脏协会 II 至 IV 级射血分数小于或等于 40% 且血浆 N 端 B 型利钠肽前体升高的患者,并进行最终随访- 2019 年 6 月 6 日更新。 干预 在推荐疗法中加入每日一次 10 毫克的达格列净或安慰剂。主要结果和测量主要结果是心力衰竭恶化或心血管死亡事件的复合。这一结果通过基线糖尿病状态进行分析,在没有糖尿病的患者中,通过糖化血红蛋白水平低于 5.7% 与大于或等于 5.7% 进行分析。结果 在随机分配的 4744 名患者中(平均年龄 66 岁;1109 名 [23%] 女性;2605 名 [55%] 没有糖尿病),4742 名完成了试验。在没有糖尿病的参与者中,主要结局发生在达格列净组 1298 名中的 171 名 (13.2%) 和安慰剂组 1307 名中的 231 名 (17.7%)(风险比,0.73 [95% CI,0.60-0.88])。在糖尿病患者中,达格列净组 1075 名患者中的 215 名 (20.0%) 和安慰剂组 1064 名患者中的 271 名 (25.5%) 发生主要结局(风险比,0.75 [95% CI,0.63-0.90])(P交互值 = .80)。在无糖尿病且糖化血红蛋白水平低于 5.7% 的患者中,主要结局发生在达格列净组 438 名患者中的 53 名 (12.1%) 和安慰剂组 419 名患者中的 71 名 (16.9%)(风险比,0.67 [95 % CI,0.47-0.96])。在糖化血红蛋白至少为 5.7% 的患者中,主要结局发生在达格列净组 860 名患者中的 118 名 (13.7%) 和安慰剂组 888 名患者中的 160 名 (18.0%)(风险比,0.74 [95% CI] , 0.59-0.94])(交互作用的 P 值 = .72)。无糖尿病患者中,达格列净组 7.3% 的患者和安慰剂组 6.1% 的患者以及糖尿病患者中 7.8% 的达格列净组患者和 7.8% 的安慰剂组患者将容量不足报告为不良事件。4 例报告了肾脏不良事件。在未患糖尿病的患者中,达格列净组中 8% 的患者和安慰剂组中 6.0% 的患者,以及糖尿病患者中达格列净组中 8.5% 的患者和安慰剂组中 8.7% 的患者。结论和相关性 在这项针对 HFrEF 患者的随机试验的探索性分析中,与安慰剂相比,当将达格列净添加到推荐治疗中时,可显着降低心力衰竭恶化或心血管死亡的风险,而与糖尿病状态无关。试验注册 ClinicalTrials.gov 标识符:NCT03036124。与安慰剂相比,dapagliflozin 加入推荐治疗后,可显着降低心力衰竭恶化或心血管死亡的风险,而与糖尿病状态无关。试验注册 ClinicalTrials.gov 标识符:NCT03036124。与安慰剂相比,dapagliflozin 添加到推荐治疗中后,可显着降低心力衰竭恶化或心血管死亡的风险,而与糖尿病状态无关。试验注册 ClinicalTrials.gov 标识符:NCT03036124。











































 京公网安备 11010802027423号
京公网安备 11010802027423号