当前位置:
X-MOL 学术
›
Mater. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synergistic effect of Nanoflower-like CdS for removal of highly toxic aqueous Cr(VI)
Materials Letters ( IF 2.7 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.matlet.2020.127734 Anurag Roy , Sasireka Velusamy , Tapas K. Mallick , Senthilarasu Sundaram
Materials Letters ( IF 2.7 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.matlet.2020.127734 Anurag Roy , Sasireka Velusamy , Tapas K. Mallick , Senthilarasu Sundaram
|
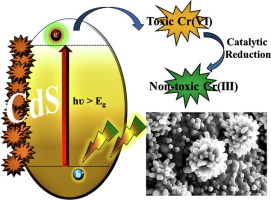
Abstract Thiourea assisted hydrothermal preparation of nanoflower-like CdS (NFs) synthesis and its efficient performance to remove toxic Cr (VI) in aqueous solution has been reported in this article. CdS NFs show synergistic behaviour via photo-catalytic reduction and adsorption to remove the toxic Cr (VI) and reduce to non-toxic Cr (III) ionic state in the aqueous solution at the room temperature. The adsorption of Cr (VI) from the aqueous solution stable time was observed ~120 min at the pH of 2 having an adsorption efficiency of 92%. Various adsorptions of kinetic and isothermal models have been further applied to understand the adsorption mechanism. The pseudo-second-order kinetic and Langmuir equilibrium adsorption isotherm model fitted best to describe Cr (VI) adsorption process. These results confirm the possibility of toxic Cr (VI) removal in water remediation without employing any costly noble metal-based catalyst.
中文翻译:

纳米花状 CdS 去除剧毒水性 Cr(VI) 的协同作用
摘要 本文报道了硫脲辅助水热制备纳米花状 CdS (NFs) 合成及其有效去除水溶液中有毒 Cr (VI) 的性能。CdS NFs 通过光催化还原和吸附表现出协同行为,以去除有毒的 Cr (VI) 并在室温下将水溶液中的 Cr (III) 离子态还原为无毒的 Cr (III)。在 pH 值为 2 时观察到从水溶液中吸附 Cr (VI) 的稳定时间约为 120 分钟,吸附效率为 92%。动力学和等温模型的各种吸附已被进一步应用于理解吸附机制。拟二级动力学和朗缪尔平衡吸附等温线模型最适合描述 Cr (VI) 吸附过程。
更新日期:2020-07-01
中文翻译:

纳米花状 CdS 去除剧毒水性 Cr(VI) 的协同作用
摘要 本文报道了硫脲辅助水热制备纳米花状 CdS (NFs) 合成及其有效去除水溶液中有毒 Cr (VI) 的性能。CdS NFs 通过光催化还原和吸附表现出协同行为,以去除有毒的 Cr (VI) 并在室温下将水溶液中的 Cr (III) 离子态还原为无毒的 Cr (III)。在 pH 值为 2 时观察到从水溶液中吸附 Cr (VI) 的稳定时间约为 120 分钟,吸附效率为 92%。动力学和等温模型的各种吸附已被进一步应用于理解吸附机制。拟二级动力学和朗缪尔平衡吸附等温线模型最适合描述 Cr (VI) 吸附过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号