当前位置:
X-MOL 学术
›
Int. J. Biol. Macromol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Designing and characterization of copper (II) ion-imprinted adsorbent based on isatin functionalized chitosan.
International Journal of Biological Macromolecules ( IF 7.7 ) Pub Date : 2020-03-27 , DOI: 10.1016/j.ijbiomac.2020.03.215 M Monier 1 , Abeer Abdulaziz H Bukhari 2 , Nadia H Elsayed 3
International Journal of Biological Macromolecules ( IF 7.7 ) Pub Date : 2020-03-27 , DOI: 10.1016/j.ijbiomac.2020.03.215 M Monier 1 , Abeer Abdulaziz H Bukhari 2 , Nadia H Elsayed 3
Affiliation
|
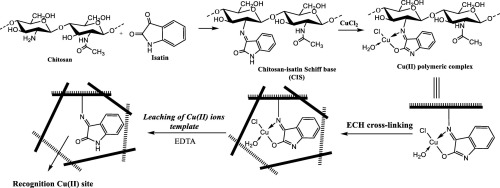
An isatin functionalized chitosan derived ion-imprinted adsorbent (Cu-CIS) was designed by tailoring Cu(II) ions imprinted cavities within the modified polysaccharide network matrix that are able to capture Cu(II) ions selectively in aqueous solution. The chelating power of chitosan toward the Cu(II) ions was first enhanced via isatin functionalization, which was cross-linked using epichlorohydrin (ECH) after loading the Cu(II) ions. The selective metal ions binding sites are then formed by eluting the coordinated Cu(II) ions using EDTA to finally produce the Cu-CIS selective sorbent. The equilibrium isotherms have been utilized to anticipate the maximum capacity of the Cu-CIS sorbent and compare it with that of the blank non-imprinted sorbent NI-CIS. In addition, the significance of inserting the Cu(II) ions recognition cavities within the adsorbent matrix was pointed out by performing the adsorption in a multi-ionic solution mixture containing Co(II), Ni(II), Pb(II), Cd(II) and Cu(II) ions and the obtained selectivity coefficients in case of Cu-CIS revealed remarkable selectivity potentials toward the Cu(II) ions compared to NI-CIS. Moreover, at the consecutive performance of a Cu-CIS absorbent for five cycles, it was found that it still held 97% of its initial capacity enabling promising applications in both water treatment and Cu (II) ions recycling.
中文翻译:

基于Isatin功能化壳聚糖的铜(II)离子印迹吸附剂的设计和表征。
通过在修饰的多糖网络基质中剪裁能够在水溶液中选择性捕获Cu(II)离子的Cu(II)离子印迹腔,设计了一种由isatin功能化的壳聚糖衍生的离子印迹吸附剂(Cu-CIS)。壳聚糖对Cu(II)离子的螯合能力首先通过isatin功能化增强,该功能在负载Cu(II)离子后使用表氯醇(ECH)进行交联。然后通过使用EDTA洗脱配位的Cu(II)离子形成选择性金属离子结合位点,最终生成Cu-CIS选择性吸附剂。平衡等温线已被用于预测Cu-CIS吸附剂的最大容量,并将其与空白非压印吸附剂NI-CIS的最大容量进行比较。此外,通过在含有Co(II),Ni(II),Pb(II),Cd(II)的多离子溶液混合物中进行吸附,指出了在吸附剂基质中插入Cu(II)离子识别腔的重要性。 Cu-II离子和Cu(II)离子以及获得的选择性系数显示出与NI-CIS相比对Cu(II)离子具有显着的选择性。而且,发现在连续五个循环的Cu-CIS吸收剂性能下,它仍保持了其初始容量的97%,从而在水处理和Cu(II)离子回收方面都有望实现应用。与NI-CIS相比,Cd(II)和Cu(II)离子以及在Cu-CIS情况下获得的选择性系数显示出对Cu(II)离子的显着选择性。而且,发现在连续五个循环的Cu-CIS吸收剂性能下,它仍保持了其初始容量的97%,从而在水处理和Cu(II)离子回收方面都有望实现应用。与NI-CIS相比,Cd(II)和Cu(II)离子以及在Cu-CIS情况下获得的选择性系数显示出对Cu(II)离子的显着选择性。而且,发现在连续五个循环的Cu-CIS吸收剂性能下,它仍保持了其初始容量的97%,从而在水处理和Cu(II)离子回收方面都有望实现应用。
更新日期:2020-03-28
中文翻译:

基于Isatin功能化壳聚糖的铜(II)离子印迹吸附剂的设计和表征。
通过在修饰的多糖网络基质中剪裁能够在水溶液中选择性捕获Cu(II)离子的Cu(II)离子印迹腔,设计了一种由isatin功能化的壳聚糖衍生的离子印迹吸附剂(Cu-CIS)。壳聚糖对Cu(II)离子的螯合能力首先通过isatin功能化增强,该功能在负载Cu(II)离子后使用表氯醇(ECH)进行交联。然后通过使用EDTA洗脱配位的Cu(II)离子形成选择性金属离子结合位点,最终生成Cu-CIS选择性吸附剂。平衡等温线已被用于预测Cu-CIS吸附剂的最大容量,并将其与空白非压印吸附剂NI-CIS的最大容量进行比较。此外,通过在含有Co(II),Ni(II),Pb(II),Cd(II)的多离子溶液混合物中进行吸附,指出了在吸附剂基质中插入Cu(II)离子识别腔的重要性。 Cu-II离子和Cu(II)离子以及获得的选择性系数显示出与NI-CIS相比对Cu(II)离子具有显着的选择性。而且,发现在连续五个循环的Cu-CIS吸收剂性能下,它仍保持了其初始容量的97%,从而在水处理和Cu(II)离子回收方面都有望实现应用。与NI-CIS相比,Cd(II)和Cu(II)离子以及在Cu-CIS情况下获得的选择性系数显示出对Cu(II)离子的显着选择性。而且,发现在连续五个循环的Cu-CIS吸收剂性能下,它仍保持了其初始容量的97%,从而在水处理和Cu(II)离子回收方面都有望实现应用。与NI-CIS相比,Cd(II)和Cu(II)离子以及在Cu-CIS情况下获得的选择性系数显示出对Cu(II)离子的显着选择性。而且,发现在连续五个循环的Cu-CIS吸收剂性能下,它仍保持了其初始容量的97%,从而在水处理和Cu(II)离子回收方面都有望实现应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号