当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structurally diverse diterpenoids from Pieris japonica as potent analgesics.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-03-27 , DOI: 10.1016/j.bioorg.2020.103794 Guijuan Zheng 1 , Pengfei Jin 1 , Lang Huang 1 , Qihua Zhang 1 , Lingkui Meng 1 , Guangmin Yao 1
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-03-27 , DOI: 10.1016/j.bioorg.2020.103794 Guijuan Zheng 1 , Pengfei Jin 1 , Lang Huang 1 , Qihua Zhang 1 , Lingkui Meng 1 , Guangmin Yao 1
Affiliation
|
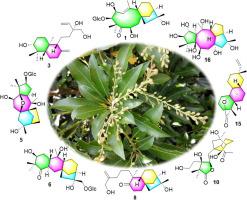
Sixteen diterpenoids (1-16) including 10 new ones, pierisjaponins A-J (1-10), were isolated and identified from Pieris japonica, and their structures were classified into eight diverse carbon skeletons. Pierisjaponins A (1) and B (2) represent the first 1,5-seco-grayanane diterpenoid glucosides and only showed 17 carbon resonances instead of 26 carbons in the 13C NMR spectra, their structures were finally defined by single-crystal X-ray diffraction, and the unusual NMR phenomena were explained. Pierisjaponin E (5) is the first mollane diterpene glucoside. This is the first time to report ent-labdane (3, 4, and 11) and ent-rosane (15) type diterpenoids from the Ericaceae plants, which provided the precursors of the Ericaceae diterpenoids and enlarged the chemical diversity of Ericaceae diterpenoids. All the 16 isolates showed potent analgesic activities, and this is the first time to describe the analgesic activities of 1,5-seco-grayanane, ent-labdane, mollane, and ent-rosane type diterpenoids. A preliminary structure-activity relationship is discussed, which provided new clues to design novel analgesics based on the Ericaceae diterpenoids.
中文翻译:

菜粉蝶结构不同的二萜作为有效的镇痛药。
从菜青虫中分离并鉴定出16种二萜类化合物(1-16),包括10种新的菜青素AJ(1-10),它们的结构分为8个不同的碳骨架。Pierisjaponins A(1)和B(2)代表第一个1,5-seco-grayanane二萜类糖苷,在13C NMR光谱中仅显示17个碳共振而不是26个碳,它们的结构最终由单晶X射线定义衍射,并解释了异常的NMR现象。Pierisjaponin E(5)是第一个烷烃二萜糖苷。这是首次报告Ericaceae植物中的恩丹丹(3、4和11)和戊烷(15)型二萜,这提供了Ericaceae二萜的前体并扩大了Ericaceae二萜的化学多样性。这16种分离物均显示出有效的镇痛活性,这是首次描述1,5-癸二草烷烷,对拉丹烷,软体动物和对戊烷型二萜的镇痛活性。初步的结构-活性关系进行了讨论,这为基于菊苣科二萜类化合物设计新颖的镇痛药提供了新的线索。
更新日期:2020-04-20
中文翻译:

菜粉蝶结构不同的二萜作为有效的镇痛药。
从菜青虫中分离并鉴定出16种二萜类化合物(1-16),包括10种新的菜青素AJ(1-10),它们的结构分为8个不同的碳骨架。Pierisjaponins A(1)和B(2)代表第一个1,5-seco-grayanane二萜类糖苷,在13C NMR光谱中仅显示17个碳共振而不是26个碳,它们的结构最终由单晶X射线定义衍射,并解释了异常的NMR现象。Pierisjaponin E(5)是第一个烷烃二萜糖苷。这是首次报告Ericaceae植物中的恩丹丹(3、4和11)和戊烷(15)型二萜,这提供了Ericaceae二萜的前体并扩大了Ericaceae二萜的化学多样性。这16种分离物均显示出有效的镇痛活性,这是首次描述1,5-癸二草烷烷,对拉丹烷,软体动物和对戊烷型二萜的镇痛活性。初步的结构-活性关系进行了讨论,这为基于菊苣科二萜类化合物设计新颖的镇痛药提供了新的线索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号