当前位置:
X-MOL 学术
›
J. Pharmaceut. Biomed. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ultra-sensitive quantification of the therapeutic cyclic peptide bremelanotide utilizing UHPLC-MS/MS for evaluation of its oral plasma pharmacokinetics.
Journal of Pharmaceutical and Biomedical Analysis ( IF 3.1 ) Pub Date : 2020-03-27 , DOI: 10.1016/j.jpba.2020.113276 Max Sauter 1 , Philipp Uhl 1 , Jürgen Burhenne 1 , Walter E Haefeli 1
Journal of Pharmaceutical and Biomedical Analysis ( IF 3.1 ) Pub Date : 2020-03-27 , DOI: 10.1016/j.jpba.2020.113276 Max Sauter 1 , Philipp Uhl 1 , Jürgen Burhenne 1 , Walter E Haefeli 1
Affiliation
|
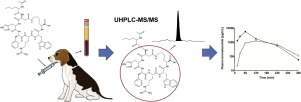
Bremelanotide (Vyleesi®), a cyclic heptapeptide, was recently approved for the subcutaneous treatment of premenopausal hypoactive sexual desire disorder. To foster the development of alternative routes of administration, we aimed at determining the oral plasma pharmacokinetics of bremelanotide in beagle dogs. Therefore, we established a UHPLC-MS/MS assay with an LLOQ of 10 pg/mL (9.8 pM) using 100 μL of plasma and validated it according to the guidelines of the US Food and Drug Administration and the European Medicines Agency. Bremelanotide was isolated from plasma by protein precipitation and quantification was performed with positive heated ESI MS/MS in the SRM mode. The calibrated concentration range of 10-10,000 pg/mL was linear showing correlation coefficients > 0.99. In the calibrated range, interday and intraday accuracy ranged from 88.9-100.0 % with corresponding precision < 8 %. Accuracy at the LLOQ ranged from 93.6-100.8 % with corresponding precision < 11 %. Because of the validity of a dilution QC that showed accurate quantification of 10-fold diluted plasma samples (accuracy 99.4 %, precision < 6 %), the assay is suitable for bremelanotide quantification in its effective concentration range up to 100,000 pg/mL. The ultra-sensitive assay was applied to the quantification of bremelanotide plasma concentrations after oral administration to beagle dogs, which indicated minimal oral absorption.
中文翻译:

利用 UHPLC-MS/MS 对治疗性环肽布雷默诺肽进行超灵敏定量,以评估其口服血浆药代动力学。
Bremelanotide (Vyleesi®) 是一种环状七肽,最近被批准用于皮下注射治疗绝经前性欲减退症。为了促进替代给药途径的开发,我们旨在确定布雷默诺肽在比格犬中的口服血浆药代动力学。因此,我们使用 100 μL 血浆建立了 LLOQ 为 10 pg/mL (9.8 pM) 的 UHPLC-MS/MS 测定,并根据美国食品和药物管理局和欧洲药品管理局的指南进行了验证。通过蛋白质沉淀从血浆中分离布雷默诺肽,并在 SRM 模式下使用正加热 ESI MS/MS 进行定量。 10-10,000 pg/mL 的校准浓度范围呈线性,相关系数 > 0.99。在校准范围内,日间和日内准确度范围为 88.9-100.0 %,相应精度为 < 8 %。 LLOQ 的准确度范围为 93.6-100.8%,相应精度为 < 11%。由于稀释 QC 的有效性显示出对 10 倍稀释血浆样品的准确定量(准确度 99.4 %,精度 < 6 %),因此该测定法适用于有效浓度范围高达 100,000 pg/mL 的布雷默诺肽定量。超灵敏测定法应用于比格犬口服给药后布雷默诺肽血浆浓度的定量,表明口服吸收极少。
更新日期:2020-03-28
中文翻译:

利用 UHPLC-MS/MS 对治疗性环肽布雷默诺肽进行超灵敏定量,以评估其口服血浆药代动力学。
Bremelanotide (Vyleesi®) 是一种环状七肽,最近被批准用于皮下注射治疗绝经前性欲减退症。为了促进替代给药途径的开发,我们旨在确定布雷默诺肽在比格犬中的口服血浆药代动力学。因此,我们使用 100 μL 血浆建立了 LLOQ 为 10 pg/mL (9.8 pM) 的 UHPLC-MS/MS 测定,并根据美国食品和药物管理局和欧洲药品管理局的指南进行了验证。通过蛋白质沉淀从血浆中分离布雷默诺肽,并在 SRM 模式下使用正加热 ESI MS/MS 进行定量。 10-10,000 pg/mL 的校准浓度范围呈线性,相关系数 > 0.99。在校准范围内,日间和日内准确度范围为 88.9-100.0 %,相应精度为 < 8 %。 LLOQ 的准确度范围为 93.6-100.8%,相应精度为 < 11%。由于稀释 QC 的有效性显示出对 10 倍稀释血浆样品的准确定量(准确度 99.4 %,精度 < 6 %),因此该测定法适用于有效浓度范围高达 100,000 pg/mL 的布雷默诺肽定量。超灵敏测定法应用于比格犬口服给药后布雷默诺肽血浆浓度的定量,表明口服吸收极少。











































 京公网安备 11010802027423号
京公网安备 11010802027423号