当前位置:
X-MOL 学术
›
J. Am. Coll. Cardiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evolocumab Use in HIV-Infected Patients With Dyslipidemia: Primary Results of the Randomized, Double-blind BEIJERINCK Study
Journal of the American College of Cardiology ( IF 21.7 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.jacc.2020.03.025 Franck Boccara 1 , Princy N Kumar 2 , Bruno Caramelli 3 , Alexandra Calmy 4 , J Antonio G López 5 , Sarah Bray 5 , Marcoli Cyrille 5 , Robert S Rosenson 6 ,
Journal of the American College of Cardiology ( IF 21.7 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.jacc.2020.03.025 Franck Boccara 1 , Princy N Kumar 2 , Bruno Caramelli 3 , Alexandra Calmy 4 , J Antonio G López 5 , Sarah Bray 5 , Marcoli Cyrille 5 , Robert S Rosenson 6 ,
Affiliation
|
BACKGROUND
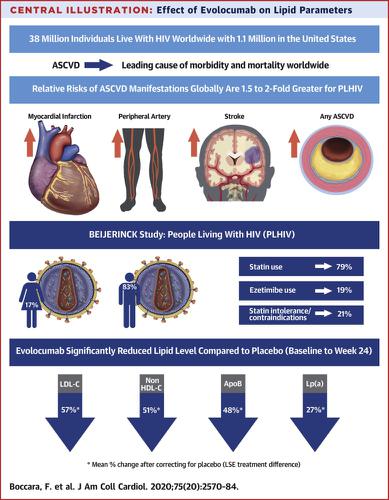
People living with HIV (PLHIV) are at increased risk of atherosclerotic cardiovascular disease (ASCVD) and prone to statin-related adverse events from drug-drug interactions with certain antiretroviral regimens. OBJECTIVES
This study sought to evaluate the efficacy and safety of evolocumab in dyslipidemic PLHIV. METHODS
BEIJERINCK is a randomized, double-blind, multinational trial comparing monthly subcutaneous evolocumab 420 mg with placebo in PLHIV with hypercholesterolemia/mixed dyslipidemia taking maximally-tolerated statin therapy. The primary endpoint was the percent change (baseline to week 24) in low-density lipoprotein cholesterol (LDL-C); secondary endpoints included achievement of LDL-C <70 mg/dL and percent change in other plasma lipid and lipoprotein levels. Treatment-emergent adverse events (TEAEs) were also examined. RESULTS
A total of 464 patients were analyzed (mean age of 56.4 years, 82.5% male, mean duration with HIV of 17.4 years). ASCVD was documented in 35.6% of patients, and statin intolerance/contraindications to statin use were present in 20.7% of patients. Evolocumab reduced LDL-C by 56.9% (95% CI: 61.6%, 52.3%) from baseline to week 24 versus placebo. An LDL-C level of <70 mg/dL was achieved in 73.3% of patients in the evolocumab group versus 7.9% in the placebo group. Evolocumab also significantly reduced other atherogenic lipid levels, including non-HDL-C, ApoB, and Lp(a) (all p<0.0001). Evolocumab was well tolerated, and TEAE patient incidence was similar among evolocumab and placebo groups. CONCLUSIONS
Evolocumab was safe and significantly reduced lipid levels in dyslipidemic PLHIV on maximally-tolerated statin therapy. Evolocumab is an effective therapy for lowering atherogenic lipoproteins in PLHIV with high cardiovascular risk.
中文翻译:

Evolocumab 在 HIV 感染的血脂异常患者中的使用:随机、双盲 BEIJERINCK 研究的主要结果
背景 HIV 感染者 (PLHIV) 患动脉粥样硬化性心血管疾病 (ASCVD) 的风险增加,并且由于与某些抗逆转录病毒疗法的药物相互作用而容易发生与他汀类药物相关的不良事件。目的 本研究旨在评估 evolocumab 在血脂异常 PLHIV 中的疗效和安全性。方法 BEIJERINCK 是一项随机、双盲、多国试验,比较每月皮下注射 evolocumab 420 mg 与安慰剂在 PLHIV 中使用最大耐受性他汀类药物治疗的高胆固醇血症/混合性血脂异常。主要终点是低密度脂蛋白胆固醇 (LDL-C) 的百分比变化(基线至第 24 周);次要终点包括实现 LDL-C <70 mg/dL 和其他血浆脂质和脂蛋白水平的百分比变化。还检查了治疗中出现的不良事件 (TEAE)。结果 共分析了 464 名患者(平均年龄 56.4 岁,男性占 82.5%,HIV 感染平均持续时间为 17.4 年)。35.6% 的患者记录了 ASCVD,20.7% 的患者存在他汀类药物不耐受/他汀类药物使用禁忌症。与安慰剂相比,Evolocumab 从基线到第 24 周使 LDL-C 降低了 56.9%(95% CI:61.6%,52.3%)。evolocumab 组 73.3% 的患者达到了 <70 mg/dL 的 LDL-C 水平,而安慰剂组为 7.9%。Evolocumab 还显着降低了其他致动脉粥样硬化的脂质水平,包括非 HDL-C、ApoB 和 Lp(a)(所有 p<0.0001)。Evolocumab 的耐受性良好,并且 Evolocumab 和安慰剂组的 TEAE 患者发生率相似。结论 Evolocumab 是安全的,并且在最大耐受他汀类药物治疗中显着降低血脂异常 PLHIV 的血脂水平。
更新日期:2020-05-01
中文翻译:

Evolocumab 在 HIV 感染的血脂异常患者中的使用:随机、双盲 BEIJERINCK 研究的主要结果
背景 HIV 感染者 (PLHIV) 患动脉粥样硬化性心血管疾病 (ASCVD) 的风险增加,并且由于与某些抗逆转录病毒疗法的药物相互作用而容易发生与他汀类药物相关的不良事件。目的 本研究旨在评估 evolocumab 在血脂异常 PLHIV 中的疗效和安全性。方法 BEIJERINCK 是一项随机、双盲、多国试验,比较每月皮下注射 evolocumab 420 mg 与安慰剂在 PLHIV 中使用最大耐受性他汀类药物治疗的高胆固醇血症/混合性血脂异常。主要终点是低密度脂蛋白胆固醇 (LDL-C) 的百分比变化(基线至第 24 周);次要终点包括实现 LDL-C <70 mg/dL 和其他血浆脂质和脂蛋白水平的百分比变化。还检查了治疗中出现的不良事件 (TEAE)。结果 共分析了 464 名患者(平均年龄 56.4 岁,男性占 82.5%,HIV 感染平均持续时间为 17.4 年)。35.6% 的患者记录了 ASCVD,20.7% 的患者存在他汀类药物不耐受/他汀类药物使用禁忌症。与安慰剂相比,Evolocumab 从基线到第 24 周使 LDL-C 降低了 56.9%(95% CI:61.6%,52.3%)。evolocumab 组 73.3% 的患者达到了 <70 mg/dL 的 LDL-C 水平,而安慰剂组为 7.9%。Evolocumab 还显着降低了其他致动脉粥样硬化的脂质水平,包括非 HDL-C、ApoB 和 Lp(a)(所有 p<0.0001)。Evolocumab 的耐受性良好,并且 Evolocumab 和安慰剂组的 TEAE 患者发生率相似。结论 Evolocumab 是安全的,并且在最大耐受他汀类药物治疗中显着降低血脂异常 PLHIV 的血脂水平。











































 京公网安备 11010802027423号
京公网安备 11010802027423号