Computational and Theoretical Chemistry ( IF 3.0 ) Pub Date : 2020-03-27 , DOI: 10.1016/j.comptc.2020.112798 Zhengguo Huang , Yajie Guo , Yuqing Li
|
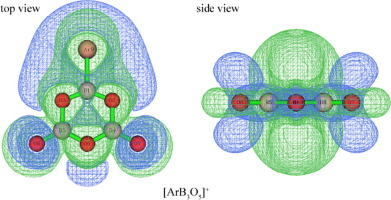
The bonds and aromaticities of [NgBxOy]+ (Ng = Ar, Kr and Xe; x = 3 ∼ 5, y = 5 ∼ 7) were studied by quantum chemistry methods. [NgBxOy]+ was confirmed to be aromatic by various methods, and the trends were proposed as follows: For compounds with the same boroxol ring, the order of aromaticity is [ArBxOy]+ > [KrBxOy]+ > [XeBxOy]+; For compounds with different boroxol ring, the order of aromaticity is [NgB3O5]+ > [NgB4O6]+ > [NgB5O7]+. The conjugation effects were found among the 3c-2e π bonds on the boroxol ring and the 3c-2e π bond on the exocyclic B-O-B branches as well as the lone electron-pair orbitals of Ng atoms. Such conjugation effects are favorable to the expansion of the delocalization region but unfavorable to aromaticity. AIM results show that the Ng-B (Ng = Kr and Xe) bonds are covalent, while the Ar-B bond except that of [ArB3O5]+ is covalent partially. The bond dissociation energy (BDE) order of Ng-B bond in compounds with the same boroxol ring is [XeBxOy]+ > [ArBxOy]+ > [KrBxOy]+, and the BDE order of Ng-B bond in compounds with different boroxol ring is [NgB3O5]+ > [NgB4O6]+ ≈ [NgB5O7]+. Therefore, [XeBxOy]+ are credible candidates for the experimental synthesis researches.
中文翻译:

[NgB x O y ] +的键和芳香性(Ng = Ar,Kr和Xe; x = 3〜5 ,y = 5〜7 )
通过量子化学方法研究了[NgB x O y ] +(Ng = Ar,Kr和Xe; x = 3〜5,y = 5〜7)的键和芳香性。[NgB x O y ] +通过多种方法证实为芳香族,其趋势如下:对于具有相同环硼氧烷环的化合物,芳香性的顺序为[ArB x O y ] + > [KrB x O y ] + > [XeB x O y ] + ; 对于具有不同环硼氧烷环的化合物,芳族顺序为[NgB 3 O 5] + > [NgB 4 O 6 ] + > [NgB 5 O 7 ] +。在硼环的3c-2eπ键和环外BOB分支的3c-2eπ键以及Ng原子的孤对电子对轨道中发现了共轭效应。这种共轭作用有利于离域区域的扩大,但不利于芳香性。AIM结果表明,Ng-B键(Ng = Kr和Xe)是共价的,而除[ArB 3 O 5 ] +以外的Ar-B键是部分共价的。具有相同硼氧杂环戊烷环的化合物中Ng-B键的键离解能(BDE)顺序为[XeB xO y ] + > [ArB x O y ] + > [KrB x O y ] +,而具有不同硼环的化合物中Ng-B键的BDE顺序为[NgB 3 O 5 ] + > [NgB 4 O 6 ] + ≈[NgB 5 O 7 ] +。因此,[XeB x O y ] +是进行实验合成研究的可靠候选者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号