Electrochemistry Communications ( IF 4.7 ) Pub Date : 2020-03-28 , DOI: 10.1016/j.elecom.2020.106706 Evgenia Dmitrieva , Xiuling Yu , Horst Hartmann
|
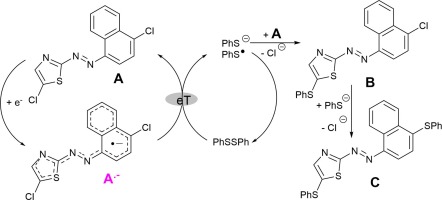
In this work, the electrochemical transformation of 5-chloro-2-[(4-chloronaphthalen-1-yl)azo]thiazoles (A) into the corresponding radical anion A·− and its subsequent reaction with diphenyldisulfide (PhSSPh) was studied. It was found that the primarily generated azo anion radical A·− is able to initiate an electron transfer process which converts the disulfide into its thiolate anion PhS−. This anion was subsequently able to substitute the Cl- and H-groups by phenylmercapto moieties in the starting azo compound A. The structures of the phenylmercapto-substituted azo compounds thus generated were confirmed by thin-layer chromatography and mass spectrometry using independently prepared compounds as references.
中文翻译:

电子转移引发的1-氯取代的4-(噻唑-2-基偶氮)萘对硫酚酸根阴离子的亲核取代
在这项工作中,研究了5-氯-2-[(4-氯萘-1-基)偶氮]噻唑(A)的电化学转化为相应的自由基阴离子A ·-以及随后与二苯基二硫键(PhSSPh)的反应。结果发现,在主要产生偶氮阴离子自由基甲· -能够发起其将二硫化物成其硫醇盐阴离子PHS的电子转移过程- 。该阴离子随后能够在起始偶氮化合物A中用苯基巯基部分取代Cl-和H-基团。。使用独立制备的化合物作为参考,通过薄层色谱法和质谱法确认了由此产生的苯基巯基取代的偶氮化合物的结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号