Tetrahedron ( IF 2.1 ) Pub Date : 2020-03-27 , DOI: 10.1016/j.tet.2020.131149 Briana R. Schrage , Victor N. Nemykin , Christopher J. Ziegler
|
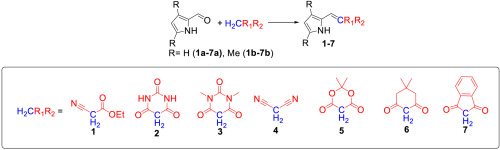
In this report, we present the synthesis and characterization of 1H-pyrrol-2-ylmethylenes, prepared from Knoevenagel condensations between pyrrole aldehydes and several organic CH acids. New compounds were characterized by a variety of spectroscopic methods, electrochemistry as well as X-ray crystallography. DFT calculations on these compounds indicates an appreciable amount of electronic delocalization between the pyrrole and acid-derived alkene units. The compounds display a single intense π→π* transitions in the UV–visible region. Ab initio and semi-empirical calculations suggest that the HOMO to LUMO single-electron excitations are responsible for the most intense absorption in new chromophores.
中文翻译:

1H-吡咯-2-基亚甲基化合物的结构和电子学
在这份报告中,我们介绍了1H-吡咯-2-基亚甲基的合成和表征,它是由吡咯醛醛与几种有机CH酸之间的Knoevenagel缩合制备的。通过多种光谱方法,电化学以及X射线晶体学对新化合物进行了表征。这些化合物的DFT计算表明,在吡咯和酸衍生的烯烃单元之间有相当数量的电子离域。这些化合物在紫外可见区域内显示出单个强烈的π→π*跃迁。从头算和半经验计算表明,HOMO到LUMO单电子激发是新发色团吸收最强的原因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号