当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cytotoxic bafilomycin analogues 6/5/5 with tricyclic ring system from a marine-derived Streptomyces sp
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-03-28 , DOI: 10.1016/j.tetlet.2020.151874 Jia-Qi Li , Hao-Wen Zhao , Zhong-Jun Ma
中文翻译:

具有海洋衍生链霉菌属物种的三环系统的细胞毒性bafilomycin类似物6/5/5
更新日期:2020-03-28
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-03-28 , DOI: 10.1016/j.tetlet.2020.151874 Jia-Qi Li , Hao-Wen Zhao , Zhong-Jun Ma
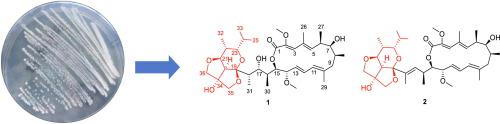
|
Two bafilomycin derivatives with an unprecedented 6/5/5 tricyclic ring system (1 and 2), along with five known analogues (3–7), were identified from a marine Streptomyces sp. The structures of 1 and 2 were elucidated by analysis of HRESIMS, extensive NMR spectroscopic methods, GIAO DET 13C NMR calculations and comparison of the spectroscopic data with those of known related compounds. Compound 2 exhibited significant cytotoxicity against PC3 human prostate carcinoma cells with IC50 value of 4.99 ± 0.15 μM.
中文翻译:

具有海洋衍生链霉菌属物种的三环系统的细胞毒性bafilomycin类似物6/5/5
以前所未有的6/5/5三环系统(两个巴弗洛霉素衍生物1和2)中,用5个已知类似物(沿3 - 7),是从海洋鉴定链霉菌属。通过HRESIMS分析,广泛的NMR光谱方法,GIAO DET 13 C NMR计算以及光谱数据与已知相关化合物的比较,阐明了1和2的结构。化合物2对PC3人前列腺癌细胞表现出明显的细胞毒性,IC 50值为4.99±0.15μM。



























 京公网安备 11010802027423号
京公网安备 11010802027423号