Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2020-03-28 , DOI: 10.1016/j.apcatb.2020.118932 Anqi Dong , Shan Gao , Xiang Wan , Linxia Wang , Tong Zhang , Li Wang , Xiuyao Lang , Weichao Wang
|
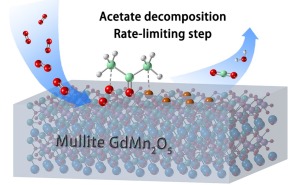
Mn-based mullite catalyst GdMn2O5 is developed via the hydrothermal method to efficiently remove volatile organic compound acetone with a high efficiency and stability in total oxidation. The T90 over GdMn2O5 approaches to 160 s°C in contrast to 191 °C over 1 wt % Pt/Al2O3. The catalytic performance maintains after reaction at 200 °C for 260 h with 5.0 % H2O. The surface labile oxygen is the key to superior catalytic performance. According to the in situ DRIFTS and the oxidation pathway proposed by DFT calculations, the acetate species decomposition is assigned as the rate-limiting step. And the calculated kinetic barrier for the rate-limiting step, 151.7 kJ/mol (1.58 eV), is in line with the apparent activation energy from acetone oxidation over GdMn2O5 of 150.0 kJ/mol (1.56 eV). This work provides insights into developing highly efficient and hydrothermally stable volatile organic compounds removal catalysts via surface oxygen activation.
中文翻译:

鲁棒的三元Mn基莫来石GdMn 2 O 5对丙酮催化氧化的不稳定氧促进作用
锰基莫来石催化剂GdMn 2 O 5通过水热法开发,可高效,高效地去除挥发性有机化合物丙酮,并具有稳定的总氧化能力。GdMn 2 O 5上的T 90接近160 s°C,而1 wt%Pt / Al 2 O 3上的191°C则相反。与5.0%H 2在200°C下反应260小时后,催化性能得以保持O.表面不稳定的氧气是获得优异催化性能的关键。根据原位DRIFTS和通过DFT计算提出的氧化途径,将乙酸酯类分解指定为限速步骤。限速步骤的动力学势垒为151.7 kJ / mol(1.58 eV),与丙酮在GdMn 2 O 5上氧化产生的表观活化能150.0 kJ / mol(1.56 eV)一致。这项工作为开发通过表面氧活化的高效和水热稳定的挥发性有机化合物去除催化剂提供了见识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号