当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cylindrical Similarity Measurement for Helices in Medium-Resolution Cryo-Electron Microscopy Density Maps.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-03-27 , DOI: 10.1021/acs.jcim.0c00010 Salim Sazzed 1 , Peter Scheible 1 , Maytha Alshammari 1 , Willy Wriggers 2 , Jing He 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-03-27 , DOI: 10.1021/acs.jcim.0c00010 Salim Sazzed 1 , Peter Scheible 1 , Maytha Alshammari 1 , Willy Wriggers 2 , Jing He 1
Affiliation
|
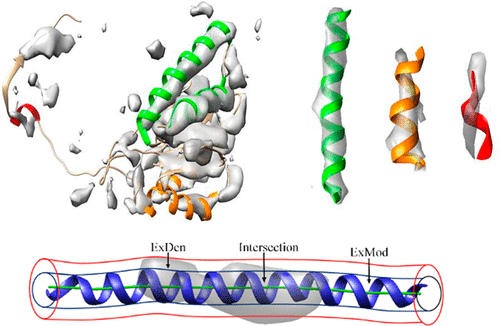
Cryo-electron microscopy (cryo-EM) density maps at medium resolution (5–10 Å) reveal secondary structural features such as α-helices and β-sheets, but they lack the side chain details that would enable a direct structure determination. Among the more than 800 entries in the Electron Microscopy Data Bank (EMDB) of medium-resolution density maps that are associated with atomic models, a wide variety of similarities can be observed between maps and models. To validate such atomic models and to classify structural features, a local similarity criterion, the F1 score, is proposed and evaluated in this study. The F1 score is theoretically normalized to a range from zero to one, providing a local measure of cylindrical agreement between the density and atomic model of a helix. A systematic scan of 30,994 helices (among 3,247 protein chains modeled into medium-resolution density maps) reveals an actual range of observed F1 scores from 0.171 to 0.848, suggesting that the cylindrical fit of the current data is well stratified by the proposed measure. The best (highest) F1 scores tend to be associated with regions that exhibit high and spatially homogeneous local resolution (between 5 Å and 7.5 Å) in the helical density. The proposed F1 scores can be used as a discriminative classifier for validation studies and as a ranking criterion for cryo-EM density features in databases.
中文翻译:

中等分辨率冷冻电子显微镜密度图中螺旋的圆柱相似性测量。
中等分辨率 (5–10 Å) 的冷冻电子显微镜 (cryo-EM) 密度图揭示了 α 螺旋和 β 折叠等二级结构特征,但它们缺乏能够直接确定结构的侧链细节。在电子显微镜数据库 (EMDB) 中与原子模型相关的中分辨率密度图的 800 多个条目中,可以观察到图和模型之间存在多种相似之处。为了验证此类原子模型并对结构特征进行分类,本研究提出并评估了局部相似性标准F 1分数。 F 1分数理论上被标准化为从 0 到 1 的范围,提供螺旋密度和原子模型之间圆柱一致性的局部测量。对 30,994 个螺旋(在建模为中等分辨率密度图的 3,247 个蛋白质链中)的系统扫描揭示了观察到的F 1分数的实际范围从 0.171 到 0.848,这表明当前数据的圆柱拟合通过所提出的测量得到了很好的分层。最佳(最高) F 1分数往往与螺旋密度中表现出高且空间均匀的局部分辨率(5 Å 至 7.5 Å 之间)的区域相关。所提出的F 1分数可用作验证研究的判别分类器以及数据库中冷冻电镜密度特征的排名标准。
更新日期:2020-03-27
中文翻译:

中等分辨率冷冻电子显微镜密度图中螺旋的圆柱相似性测量。
中等分辨率 (5–10 Å) 的冷冻电子显微镜 (cryo-EM) 密度图揭示了 α 螺旋和 β 折叠等二级结构特征,但它们缺乏能够直接确定结构的侧链细节。在电子显微镜数据库 (EMDB) 中与原子模型相关的中分辨率密度图的 800 多个条目中,可以观察到图和模型之间存在多种相似之处。为了验证此类原子模型并对结构特征进行分类,本研究提出并评估了局部相似性标准F 1分数。 F 1分数理论上被标准化为从 0 到 1 的范围,提供螺旋密度和原子模型之间圆柱一致性的局部测量。对 30,994 个螺旋(在建模为中等分辨率密度图的 3,247 个蛋白质链中)的系统扫描揭示了观察到的F 1分数的实际范围从 0.171 到 0.848,这表明当前数据的圆柱拟合通过所提出的测量得到了很好的分层。最佳(最高) F 1分数往往与螺旋密度中表现出高且空间均匀的局部分辨率(5 Å 至 7.5 Å 之间)的区域相关。所提出的F 1分数可用作验证研究的判别分类器以及数据库中冷冻电镜密度特征的排名标准。










































 京公网安备 11010802027423号
京公网安备 11010802027423号