当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-catalyzed oxidation of β-C(sp3)-H bond of primary alkylamines through a rare four-membered palladacycle intermediate
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-27 , DOI: 10.1021/jacs.0c01629 Bo Su 1 , Ala Bunescu 1 , Yehao Qiu 1 , Stephan J Zuend 2 , Martin Ernst 3 , John F Hartwig 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-27 , DOI: 10.1021/jacs.0c01629 Bo Su 1 , Ala Bunescu 1 , Yehao Qiu 1 , Stephan J Zuend 2 , Martin Ernst 3 , John F Hartwig 1
Affiliation
|
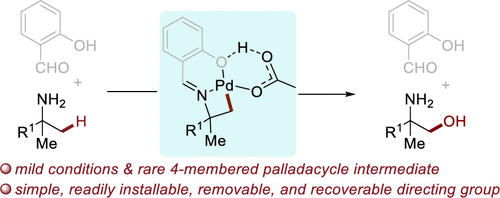
Site-selective functionalizations of C-H bonds are often achieved with a directing group that leads to five- or six-membered metallacyclic intermediates. Analogous reactions that occur through four-membered metallacycles are rare. We report a challenging palladium-catalyzed oxidation of primary C-H bonds β to nitrogen in an imine of an aliphatic amine, a process that occurs through a four-membered palladacyclc intermediate. The success of the reaction relies on the identification, by a study on H/D exchange, of a simple directing group (salicylaldehyde) capable of inducing the formation of this small ring. To gain insight into the mechanism of this unusual oxidation reaction, a series of mechanistic experiments and DFT calculations were conducted. The experimental studies showed that cleavage of the C-H bond is rate-limiting, and formation of the strained four-membered palladacycle is thermodynamically uphill. DFT calculations corroborated these conclusions and suggested that the presence of an intramolecular hydrogen bond between the oxygen of the directing group and hydroxyl group of the ligating acetic acid that is crucial for stabilization of the palladacyclic intermediate.
中文翻译:

钯催化通过罕见的四元钯环中间体氧化伯烷基胺的 β-C(sp3)-H 键
CH 键的位点选择性官能化通常通过导向基团实现,从而产生五元或六元金属环中间体。通过四元金属环发生的类似反应很少见。我们报告了一种具有挑战性的钯催化脂肪胺亚胺中伯 CH 键 β 与氮的氧化,该过程通过四元环钯中间体发生。该反应的成功依赖于通过 H/D 交换研究鉴定出能够诱导该小环形成的简单导向基团(水杨醛)。为了深入了解这种不寻常的氧化反应的机制,进行了一系列机械实验和 DFT 计算。实验研究表明,CH 键的断裂是速率限制的,并且应变四元环的形成在热力学上是艰难的。DFT 计算证实了这些结论,并表明导向基团的氧和连接乙酸的羟基之间存在分子内氢键,这对于环环中间体的稳定至关重要。
更新日期:2020-03-27
中文翻译:

钯催化通过罕见的四元钯环中间体氧化伯烷基胺的 β-C(sp3)-H 键
CH 键的位点选择性官能化通常通过导向基团实现,从而产生五元或六元金属环中间体。通过四元金属环发生的类似反应很少见。我们报告了一种具有挑战性的钯催化脂肪胺亚胺中伯 CH 键 β 与氮的氧化,该过程通过四元环钯中间体发生。该反应的成功依赖于通过 H/D 交换研究鉴定出能够诱导该小环形成的简单导向基团(水杨醛)。为了深入了解这种不寻常的氧化反应的机制,进行了一系列机械实验和 DFT 计算。实验研究表明,CH 键的断裂是速率限制的,并且应变四元环的形成在热力学上是艰难的。DFT 计算证实了这些结论,并表明导向基团的氧和连接乙酸的羟基之间存在分子内氢键,这对于环环中间体的稳定至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号