当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the 1,3-Dipolar Cycloadditions of Allenes.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-03-27 , DOI: 10.1002/chem.202000857 Song Yu 1 , Pascal Vermeeren 1 , Kevin van Dommelen 1 , F Matthias Bickelhaupt 1, 2 , Trevor A Hamlin 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-03-27 , DOI: 10.1002/chem.202000857 Song Yu 1 , Pascal Vermeeren 1 , Kevin van Dommelen 1 , F Matthias Bickelhaupt 1, 2 , Trevor A Hamlin 1
Affiliation
|
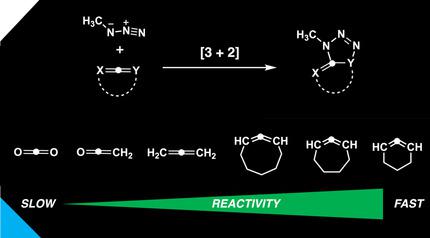
We have quantum chemically studied the reactivity, site‐, and regioselectivity of the 1,3‐dipolar cycloaddition between methyl azide and various allenes, including the archetypal allene propadiene, heteroallenes, and cyclic allenes, by using density functional theory (DFT). The 1,3‐dipolar cycloaddition reactivity of linear (hetero)allenes decreases as the number of heteroatoms in the allene increases, and formation of the 1,5‐adduct is, in all cases, favored over the 1,4‐adduct. Both effects find their origin in the strength of the primary orbital interactions. The cycloaddition reactivity of cyclic allenes was also investigated, and the increased predistortion of allenes, that results upon cyclization, leads to systematically lower activation barriers not due to the expected variations in the strain energy, but instead from the differences in the interaction energy. The geometric predistortion of cyclic allenes enhances the reactivity compared to linear allenes through a unique mechanism that involves a smaller HOMO–LUMO gap, which manifests as more stabilizing orbital interactions.
中文翻译:

了解艾伦烯的 1,3-偶极环加成。
我们利用密度泛函理论 (DFT),从量子化学角度研究了甲基叠氮化物与各种丙二烯(包括典型的丙二烯、杂丙二烯和环状丙二烯)之间 1,3-偶极环加成的反应性、位点和区域选择性。线性(杂)丙二烯的 1,3-偶极环加成反应性随着丙二烯中杂原子数量的增加而降低,并且在所有情况下,1,5-加合物的形成都优于 1,4-加合物。这两种效应都源于主轨道相互作用的强度。还研究了环状丙二烯的环加成反应性,并且环化时导致的丙二烯预变形增加导致系统性地降低活化势垒,这不是由于应变能的预期变化,而是由于相互作用能的差异。与线性丙二烯相比,环状丙二烯的几何预畸变通过一种独特的机制增强了反应性,该机制涉及更小的 HOMO-LUMO 间隙,这表现为更稳定的轨道相互作用。
更新日期:2020-03-27
中文翻译:

了解艾伦烯的 1,3-偶极环加成。
我们利用密度泛函理论 (DFT),从量子化学角度研究了甲基叠氮化物与各种丙二烯(包括典型的丙二烯、杂丙二烯和环状丙二烯)之间 1,3-偶极环加成的反应性、位点和区域选择性。线性(杂)丙二烯的 1,3-偶极环加成反应性随着丙二烯中杂原子数量的增加而降低,并且在所有情况下,1,5-加合物的形成都优于 1,4-加合物。这两种效应都源于主轨道相互作用的强度。还研究了环状丙二烯的环加成反应性,并且环化时导致的丙二烯预变形增加导致系统性地降低活化势垒,这不是由于应变能的预期变化,而是由于相互作用能的差异。与线性丙二烯相比,环状丙二烯的几何预畸变通过一种独特的机制增强了反应性,该机制涉及更小的 HOMO-LUMO 间隙,这表现为更稳定的轨道相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号