当前位置:
X-MOL 学术
›
Environ. Sci.: Processes Impacts
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interactions of emerging contaminants with model colloidal microplastics, C60 fullerene, and natural organic matter - effect of surface functional group and adsorbate properties.
Environmental Science: Processes & Impacts ( IF 4.3 ) Pub Date : 2020-03-27 , DOI: 10.1039/d0em00026d Tyler Williams 1 , Clare Walsh , Keith Murray , Mahamud Subir
Environmental Science: Processes & Impacts ( IF 4.3 ) Pub Date : 2020-03-27 , DOI: 10.1039/d0em00026d Tyler Williams 1 , Clare Walsh , Keith Murray , Mahamud Subir
Affiliation
|
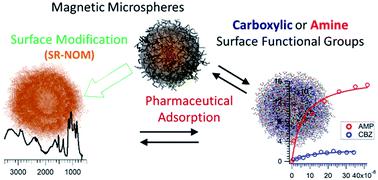
Surface adsorption of two commonly detected emerging contaminants, amlodipine (AMP) and carbamazepine (CBZ), onto model colloidal microplastics, natural organic matter (NOM), and fullerene nanomaterials have been investigated. It is found that AMP accumulation at these colloidal–aqueous interfaces is markedly higher than that of CBZ. Measurements of surface excess and particle zeta potential, along with pH-dependent adsorption studies, reveal a distinct influence of colloidal functional group on the adsorption properties of these pharmaceuticals. AMP shows a clear preference for a surface containing carboxylic group compared to an amine modified surface. CBZ, in contrast, exhibit a pH-dependent surface proclivity for both of these microparticles. The type of interactions and molecular differences with respect to structural rigidity and charge properties explain these observed behaviors. In this work, we also demonstrate a facile approach in fabricating uniform microspheres coated with NOM and C60 nanoclusters. Subsequent binding studies on these surfaces show considerable adsorption on the NOM surface but a minimal uptake of CBZ by C60. Adsorption induced colloidal aggregation was not observed. These findings map out the extent of contaminant removal by colloids of different surface properties available in the aquatic environment. The methodology developed for the adsorption study also opens up the possibility for further investigations into colloidal–contaminant interactions.
中文翻译:

新兴污染物与模型胶体微塑料,C60富勒烯和天然有机物的相互作用-表面官能团和吸附质的影响。
已经研究了两种常见的新兴污染物氨氯地平(AMP)和卡马西平(CBZ)在模型胶体微塑料,天然有机物(NOM)和富勒烯纳米材料上的表面吸附。发现在这些胶体-水界面的AMP积累明显高于CBZ。对表面过量和颗粒zeta电位的测量以及pH依赖性吸附研究表明,胶体官能团对这些药物的吸附性能有明显的影响。与胺改性的表面相比,AMP显示出对含羧基表面的明显偏好。相比之下,CBZ对这两个微粒都表现出pH依赖的表面特性。关于结构刚性和电荷性质的相互作用类型和分子差异解释了这些观察到的行为。在这项工作中,我们还展示了一种简便的方法,可用于制备涂有NOM和C的均匀微球60个纳米簇。随后在这些表面上的结合研究表明,NOM表面有大量吸附,但C 60对CBZ的吸收最少。没有观察到吸附诱导的胶体聚集。这些发现勾勒出了水生环境中可利用的具有不同表面特性的胶体去除污染物的程度。为吸附研究开发的方法学也为进一步研究胶体-污染物相互作用提供了可能性。
更新日期:2020-03-27
中文翻译:

新兴污染物与模型胶体微塑料,C60富勒烯和天然有机物的相互作用-表面官能团和吸附质的影响。
已经研究了两种常见的新兴污染物氨氯地平(AMP)和卡马西平(CBZ)在模型胶体微塑料,天然有机物(NOM)和富勒烯纳米材料上的表面吸附。发现在这些胶体-水界面的AMP积累明显高于CBZ。对表面过量和颗粒zeta电位的测量以及pH依赖性吸附研究表明,胶体官能团对这些药物的吸附性能有明显的影响。与胺改性的表面相比,AMP显示出对含羧基表面的明显偏好。相比之下,CBZ对这两个微粒都表现出pH依赖的表面特性。关于结构刚性和电荷性质的相互作用类型和分子差异解释了这些观察到的行为。在这项工作中,我们还展示了一种简便的方法,可用于制备涂有NOM和C的均匀微球60个纳米簇。随后在这些表面上的结合研究表明,NOM表面有大量吸附,但C 60对CBZ的吸收最少。没有观察到吸附诱导的胶体聚集。这些发现勾勒出了水生环境中可利用的具有不同表面特性的胶体去除污染物的程度。为吸附研究开发的方法学也为进一步研究胶体-污染物相互作用提供了可能性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号