当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction of water with oligo(ethylene glycol) terminated monolayers: wetting versus hydration
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020/03/27 , DOI: 10.1039/d0cp00906g Mustafa Sayin 1, 2, 3, 4 , Alexei Nefedov 4, 5, 6, 7 , Michael Zharnikov 1, 2, 3, 4
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020/03/27 , DOI: 10.1039/d0cp00906g Mustafa Sayin 1, 2, 3, 4 , Alexei Nefedov 4, 5, 6, 7 , Michael Zharnikov 1, 2, 3, 4
Affiliation
|
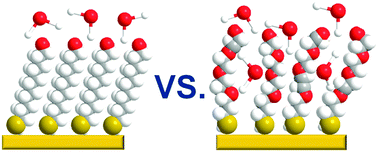
Biorepulsivity of oligo(ethylene glycol) (OEG) substituted self-assembled monolayers (SAMs), serving as model systems for analogous polymeric surfaces, is generally ascribed to the hydration effect. In this context, we applied temperature-programmed desorption to study interaction of water (D2O) with a series of OH-terminated, OEG-substituted alkanethiolate SAMs with variable length of the OEG strand, defining their biorepulsion behavior. Along with the ice overlayer (wetting phase), growing also on the surface of the analogous non-substituted films, a hydration phase, corresponding to the adsorption of D2O into the OEG matrix, was observed, with a higher desorption energy (12.4 kcal mol−1vs. 10.4 kcal mol−1) and a weight correlating with the length of the OEG strand and, consequently, with biorepulsivity. The formation of hydration phase was found to occur over an activation barrier, presumably by temperature-promoted diffusion from the wetting phase, with this process being additionally enforced by a pre-desorption annealing.
中文翻译:

水与低聚乙二醇末端单层的相互作用:润湿与水合
用作类似聚合物表面模型系统的低聚乙二醇(OEG)取代的自组装单分子层(SAMs)的生物排斥性通常归因于其水合作用。在这种情况下,我们应用程序升温脱附来研究水(D 2 O)与一系列OH末端,OEG取代的链烷硫醇盐SAM的相互作用,其中OEG链的长度可变,从而定义了它们的生物排斥行为。伴随着冰覆层(润湿相),在类似的非取代膜表面也生长,观察到水合相,对应于D 2 O吸附在OEG基质中,具有较高的解吸能(12.4) kcal mol -1与10.4 kcal mol -1)和与OEG链的长度相关的重量,因此与生物排斥性相关。发现水合相的形成发生在活化屏障上,这大概是由于温度促进了从润湿相的扩散,而该过程还通过预解吸退火来强制实施。
更新日期:2020-04-15
中文翻译:

水与低聚乙二醇末端单层的相互作用:润湿与水合
用作类似聚合物表面模型系统的低聚乙二醇(OEG)取代的自组装单分子层(SAMs)的生物排斥性通常归因于其水合作用。在这种情况下,我们应用程序升温脱附来研究水(D 2 O)与一系列OH末端,OEG取代的链烷硫醇盐SAM的相互作用,其中OEG链的长度可变,从而定义了它们的生物排斥行为。伴随着冰覆层(润湿相),在类似的非取代膜表面也生长,观察到水合相,对应于D 2 O吸附在OEG基质中,具有较高的解吸能(12.4) kcal mol -1与10.4 kcal mol -1)和与OEG链的长度相关的重量,因此与生物排斥性相关。发现水合相的形成发生在活化屏障上,这大概是由于温度促进了从润湿相的扩散,而该过程还通过预解吸退火来强制实施。











































 京公网安备 11010802027423号
京公网安备 11010802027423号