Nature Communications ( IF 14.7 ) Pub Date : 2020-03-27 , DOI: 10.1038/s41467-020-15392-9 Clément Madru 1 , Ghislaine Henneke 2 , Pierre Raia 1, 3 , Inès Hugonneau-Beaufet 1 , Gérard Pehau-Arnaudet 4 , Patrick England 5 , Erik Lindahl 6, 7 , Marc Delarue 1 , Marta Carroni 6 , Ludovic Sauguet 1
|
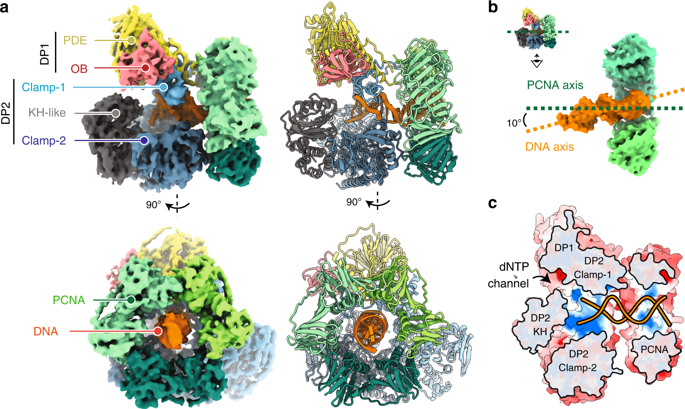
Replicative DNA polymerases (DNAPs) have evolved the ability to copy the genome with high processivity and fidelity. In Eukarya and Archaea, the processivity of replicative DNAPs is greatly enhanced by its binding to the proliferative cell nuclear antigen (PCNA) that encircles the DNA. We determined the cryo-EM structure of the DNA-bound PolD–PCNA complex from Pyrococcus abyssi at 3.77 Å. Using an integrative structural biology approach — combining cryo-EM, X-ray crystallography, protein–protein interaction measurements, and activity assays — we describe the molecular basis for the interaction and cooperativity between a replicative DNAP and PCNA. PolD recruits PCNA via a complex mechanism, which requires two different PIP-boxes. We infer that the second PIP-box, which is shared with the eukaryotic Polα replicative DNAP, plays a dual role in binding either PCNA or primase, and could be a master switch between an initiation and a processive phase during replication.
中文翻译:

与PCNA结合的D家族DNA聚合酶持续合成能力提高的结构基础。
复制性DNA聚合酶(DNAP)已发展出以高生产力和保真度复制基因组的能力。在Eukarya和Archaea中,复制性DNAPs通过与环绕DNA的增殖细胞核抗原(PCNA)结合而大大提高了其合成能力。我们确定了深渊热球菌DNA结合的PolD-PCNA复合物的冷冻EM结构。在3.77Å。使用整合的结构生物学方法-结合了冷冻EM,X射线晶体学,蛋白质-蛋白质相互作用测量和活性测定-我们描述了复制型DNAP和PCNA之间相互作用和协同作用的分子基础。PolD通过复杂的机制招募PCNA,这需要两个不同的PIP框。我们推断,与真核Polα复制DNAP共享的第二个PIP盒在结合PCNA或引物酶方面起着双重作用,并且可能是复制过程中起始阶段和过程阶段之间的主要开关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号