Scientific Reports ( IF 3.8 ) Pub Date : 2020-03-27 , DOI: 10.1038/s41598-020-62272-9 Monika Sharma 1 , Ludovic Boytard 2 , Tarik Hadi 2 , Graeme Koelwyn 1 , Russell Simon 1 , Mireille Ouimet 1 , Lena Seifert 2 , Westley Spiro 1 , Bo Yan 1 , Susan Hutchison 1 , Edward A Fisher 1 , Ravichandran Ramasamy 3 , Bhama Ramkhelawon 2 , Kathryn J Moore 1
|
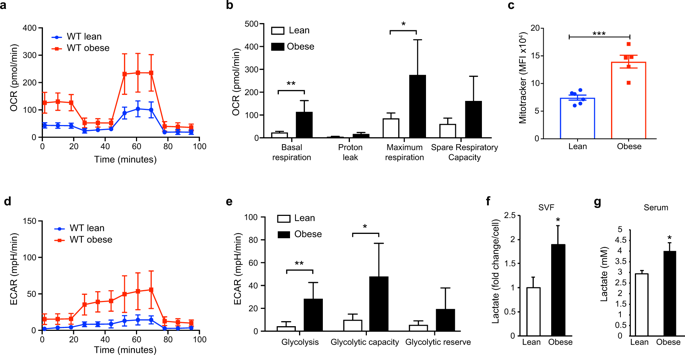
During obesity, macrophages infiltrate the visceral adipose tissue and promote inflammation that contributes to type II diabetes. Evidence suggests that the rewiring of cellular metabolism can regulate macrophage function. However, the metabolic programs that characterize adipose tissue macrophages (ATM) in obesity are poorly defined. Here, we demonstrate that ATM from obese mice exhibit metabolic profiles characterized by elevated glycolysis and oxidative phosphorylation, distinct from ATM from lean mice. Increased activation of HIF-1α in ATM of obese visceral adipose tissue resulted in induction of IL-1β and genes in the glycolytic pathway. Using a hypoxia-tracer, we show that HIF-1α nuclear translocation occurred both in hypoxic and non-hypoxic ATM suggesting that both hypoxic and pseudohypoxic stimuli activate HIF-1α and its target genes in ATM during diet-induced obesity. Exposure of macrophages to the saturated fatty acid palmitate increased glycolysis and HIF-1α expression, which culminated in IL-1β induction thereby simulating pseudohypoxia. Using mice with macrophage-specific targeted deletion of HIF-1α, we demonstrate the critical role of HIF-1α-derived from macrophages in regulating ATM accumulation, and local and systemic IL-1β production, but not in modulating systemic metabolic responses. Collectively, our data identify enhanced glycolysis and HIF-1α activation as drivers of low-grade inflammation in obesity.
中文翻译:

肥胖组织巨噬细胞中增强的糖酵解和HIF-1α活化维持肥胖症中局部和全身性白介素1β的产生。
在肥胖过程中,巨噬细胞会渗入内脏脂肪组织并促进炎症,导致II型糖尿病。有证据表明,细胞代谢的重新连接可以调节巨噬细胞功能。但是,在肥胖症中表征脂肪组织巨噬细胞(ATM)的代谢程序定义不明确。在这里,我们证明了肥胖小鼠的ATM表现出以糖酵解和氧化磷酸化升高为特征的代谢谱,这与瘦小鼠的ATM不同。肥胖内脏脂肪组织的ATM中HIF-1α的激活增加,导致IL-1β和糖酵解途径中的基因被诱导。使用缺氧示踪剂 我们表明,HIF-1α核易位发生在低氧和非低氧的ATM中,这表明低氧和假低氧刺激物在饮食诱导的肥胖过程中均激活了ATM中的HIF-1α及其靶基因。巨噬细胞暴露于饱和脂肪酸棕榈酸酯可增加糖酵解和HIF-1α表达,最终导致IL-1β诱导,从而模拟假性低氧。使用具有巨噬细胞特异性靶向缺失HIF-1α的小鼠,我们证明了巨噬细胞衍生的HIF-1α在调节ATM积累以及局部和全身性IL-1β产生中的关键作用,但在调节全身性代谢反应中没有关键作用。总体而言,我们的数据确定糖酵解和HIF-1α活化增强是肥胖症中低度炎症的驱动因素。巨噬细胞暴露于饱和脂肪酸棕榈酸酯可增加糖酵解和HIF-1α表达,最终导致IL-1β诱导,从而模拟假性低氧。使用具有巨噬细胞特异性靶向缺失HIF-1α的小鼠,我们证明了巨噬细胞衍生的HIF-1α在调节ATM积累以及局部和全身性IL-1β产生中的关键作用,但在调节全身性代谢反应中没有关键作用。总体而言,我们的数据确定糖酵解和HIF-1α活化增强是肥胖症中低度炎症的驱动因素。巨噬细胞暴露于饱和脂肪酸棕榈酸酯可增加糖酵解和HIF-1α表达,最终导致IL-1β诱导,从而模拟假性低氧。使用具有巨噬细胞特异性靶向缺失HIF-1α的小鼠,我们证明了巨噬细胞衍生的HIF-1α在调节ATM积累以及局部和全身性IL-1β产生中的关键作用,但在调节全身性代谢反应中没有关键作用。总体而言,我们的数据确定糖酵解和HIF-1α活化增强是肥胖症中低度炎症的驱动因素。和局部和全身性IL-1β的产生,但不能调节全身性代谢反应。总体而言,我们的数据确定糖酵解和HIF-1α活化增强是肥胖症中低度炎症的驱动因素。和局部和全身性IL-1β的产生,但不能调节全身性代谢反应。总体而言,我们的数据确定糖酵解和HIF-1α活化增强是肥胖症中低度炎症的驱动因素。









































 京公网安备 11010802027423号
京公网安备 11010802027423号