Scientific Reports ( IF 3.8 ) Pub Date : 2020-03-27 , DOI: 10.1038/s41598-020-62530-w Maria Dumitrascuta 1 , Marcel Bermudez 2 , Tanila Ben Haddou 1 , Elena Guerrieri 1 , Lea Schläfer 1 , Andreas Ritsch 1 , Sandor Hosztafi 3 , Aquilino Lantero 1 , Christoph Kreutz 4 , Dominique Massotte 5 , Helmut Schmidhammer 1 , Gerhard Wolber 2 , Mariana Spetea 1
|
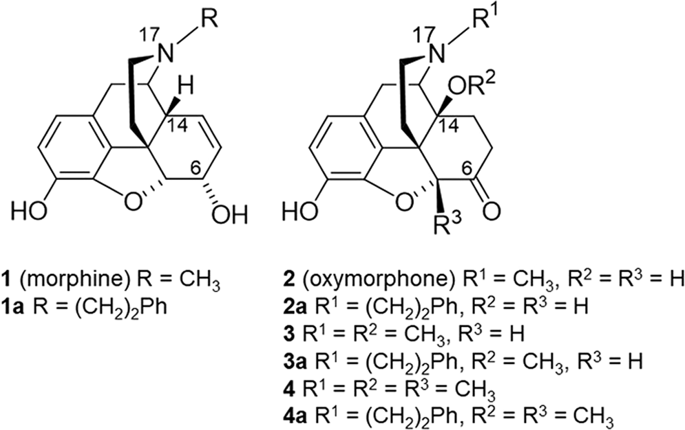
Morphine and structurally-derived compounds are µ opioid receptor (µOR) agonists, and the most effective analgesic drugs. However, their usefulness is limited by serious side effects, including dependence and abuse potential. The N-substituent in morphinans plays an important role in opioid activities in vitro and in vivo. This study presents the synthesis and pharmacological evaluation of new N-phenethyl substituted 14-O-methylmorphinan-6-ones. Whereas substitution of the N-methyl substituent in morphine (1) and oxymorphone (2) by an N-phenethyl group enhances binding affinity, selectivity and agonist potency at the µOR of 1a and 2a, the N-phenethyl substitution in 14-methoxy-N-methylmorphinan-6-ones (3 and 4) converts selective µOR ligands into dual µ/δOR agonists (3a and 4a). Contrary to N-methylmorphinans 1–4, the N-phenethyl substituted morphinans 1a–4a produce effective and potent antinociception without motor impairment in mice. Using docking and molecular dynamics simulations with the µOR, we establish that N-methylmorphinans 1–4 and their N-phenethyl counterparts 1a–4a share several essential receptor-ligand interactions, but also interaction pattern differences related to specific structural features, thus providing a structural basis for their pharmacological profiles. The emerged structure-activity relationships in this class of morphinans provide important information for tuning in vitro and in vivo opioid activities towards discovery of effective and safer analgesics.
中文翻译:

14-Methoxy-N-methylmorphinan-6-ones 中的 N-苯乙基取代将选择性 µ 阿片受体配体转化为双 µ/δ 阿片受体激动剂。
吗啡和结构衍生的化合物是 µ 阿片受体 (µOR) 激动剂,也是最有效的镇痛药物。然而,它们的有用性受到严重副作用的限制,包括依赖和滥用的可能性。吗啡喃中的 N-取代基在体外和体内阿片类药物活性中起重要作用。本研究介绍了新型N-苯乙基取代的 14 - O -methylmorphinan-6-ones 的合成和药理学评价。而吗啡 ( 1 ) 和羟吗啡酮 ( 2 )中的N-甲基取代基被N-苯乙基基团取代可增强结合亲和力、选择性和激动剂效力如图 1a和2a所示,14-甲氧基-N-甲基吗啡喃-6-酮(3和4 )中的N-苯乙基取代将选择性 µOR 配体转化为双 µ/δOR 激动剂(3a和4a)。与N-甲基吗啡喃1 – 4相反,N-苯乙基取代的吗啡喃1a – 4a在小鼠中产生有效和强效的镇痛作用,而没有运动障碍。使用 µOR 的对接和分子动力学模拟,我们确定N-甲基吗啡喃1-4及其N-苯乙基对应物1a - 4a共享几个重要的受体-配体相互作用,但也与特定结构特征相关的相互作用模式差异,从而为其药理学特征提供结构基础。这类吗啡喃中出现的构效关系为调整体外和体内阿片类药物活性以发现有效和更安全的镇痛剂提供了重要信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号