npj Biofilms and Microbiomes ( IF 7.8 ) Pub Date : 2020-03-27 , DOI: 10.1038/s41522-020-0125-2 Agustina Taglialegna 1 , Leticia Matilla-Cuenca 1, 2 , Pedro Dorado-Morales 2 , Susanna Navarro 3 , Salvador Ventura 3 , James A Garnett 4 , Iñigo Lasa 2 , Jaione Valle 1
|
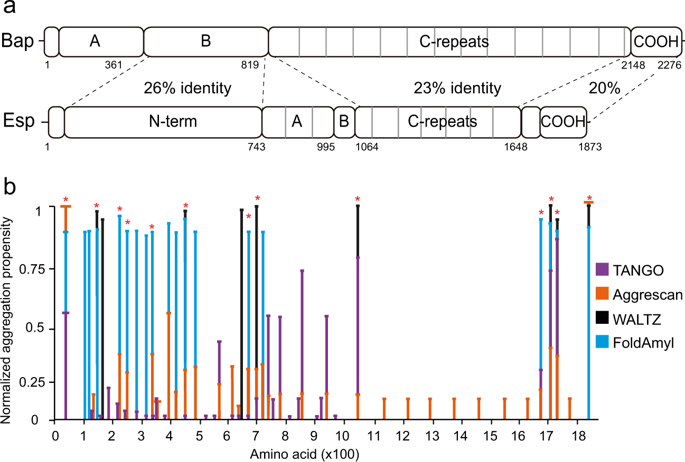
Functional amyloids are considered as common building block structures of the biofilm matrix in different bacteria. In previous work, we have shown that the staphylococcal surface protein Bap, a member of the Biofilm-Associated Proteins (BAP) family, is processed and the fragments containing the N-terminal region become aggregation-prone and self-assemble into amyloid-like structures. Here, we report that Esp, a Bap-orthologous protein produced by Enterococcus faecalis, displays a similar amyloidogenic behavior. We demonstrate that at acidic pH the N-terminal region of Esp forms aggregates with an amyloid-like conformation, as evidenced by biophysical analysis and the binding of protein aggregates to amyloid-indicative dyes. Expression of a chimeric protein, with its Esp N-terminal domain anchored to the cell wall through the R domain of clumping factor A, showed that the Esp N-terminal region is sufficient to confer multicellular behavior through the formation of an extracellular amyloid-like material. These results suggest that the mechanism of amyloid-like aggregation to build the biofilm matrix might be widespread among BAP-like proteins. This amyloid-based mechanism may not only have strong relevance for bacteria lifestyle but could also contribute to the amyloid burden to which the human physiology is potentially exposed.
中文翻译:

粪肠球菌的生物膜相关表面蛋白Esp形成淀粉样纤维。
功能性淀粉样蛋白被认为是不同细菌中生物膜基质的常见构件。在以前的工作中,我们已经显示了葡萄球菌表面蛋白Bap(生物膜相关蛋白(BAP)家族的成员)经过处理,并且包含N端区域的片段变得易于聚集并自组装成淀粉样蛋白结构。在这里,我们报道Esp,由肠球菌产生的Bap直系同源蛋白,显示相似的淀粉样蛋白生成行为。我们证明,在酸性pH下,Esp的N端区域形成具有淀粉样样构象的聚集体,这是通过生物物理分析和蛋白质聚集体与淀粉样指示染料的结合所证明的。嵌合蛋白的表达,其Esp N末端结构域通过聚集因子A的R结构域锚定到细胞壁,表明Esp N末端区域足以通过形成细胞外淀粉样样赋予多细胞行为。材料。这些结果表明,淀粉样蛋白样聚集体构建生物膜基质的机制可能在BAP样蛋白中广泛存在。










































 京公网安备 11010802027423号
京公网安备 11010802027423号