当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of triphenylene-fused phosphole oxides via C–H functionalizations
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-03-27 , DOI: 10.3762/bjoc.16.48 Md Shafiqur Rahman , Naohiko Yoshikai
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-03-27 , DOI: 10.3762/bjoc.16.48 Md Shafiqur Rahman , Naohiko Yoshikai
|
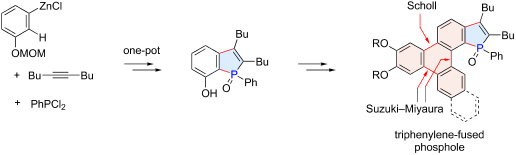
The synthesis of triphenylene-fused phosphole oxides has been achieved through two distinct C–H functionalization reactions as key steps. The phosphole ring was constructed by a three-component coupling of 3-(methoxymethoxy)phenylzinc chloride, an alkyne, and dichlorophenylphosphine, involving the regioselective C–H activation of the C2 position of the arylzinc intermediate via 1,4-cobalt migration. The resulting 7-hydroxybenzo[b]phosphole derivative was used for further π-extension through Suzuki–Miyaura couplings and a Scholl reaction, the latter closing the triphenylene ring. The absorption and emission spectra of the thus-synthesized compounds illustrated their nature as hybrids of triphenylene and benzo[b]phosphole.
中文翻译:

通过C–H官能化合成三亚苯基稠合的磷氧化物
通过两个不同的C–H官能化反应作为关键步骤,实现了三亚苯基稠合的氧化磷的合成。通过3-(甲氧基甲氧基)苯基氯化锌,炔烃和二氯苯基膦的三组分偶联来构建磷脂环,涉及通过1,4-钴迁移对芳基锌中间体的C2位进行区域选择性的CH活化。生成的7-羟基苯并[ b ]磷酸酯衍生物通过Suzuki-Miyaura偶联和Scholl反应用于进一步的π延伸,后者关闭了亚苯撑环。如此合成的化合物的吸收和发射光谱说明了它们作为三亚苯基和苯并[ b ]磷的杂化物的性质。
更新日期:2020-03-27
中文翻译:

通过C–H官能化合成三亚苯基稠合的磷氧化物
通过两个不同的C–H官能化反应作为关键步骤,实现了三亚苯基稠合的氧化磷的合成。通过3-(甲氧基甲氧基)苯基氯化锌,炔烃和二氯苯基膦的三组分偶联来构建磷脂环,涉及通过1,4-钴迁移对芳基锌中间体的C2位进行区域选择性的CH活化。生成的7-羟基苯并[ b ]磷酸酯衍生物通过Suzuki-Miyaura偶联和Scholl反应用于进一步的π延伸,后者关闭了亚苯撑环。如此合成的化合物的吸收和发射光谱说明了它们作为三亚苯基和苯并[ b ]磷的杂化物的性质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号