当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regio- and stereoselective synthesis of new ensembles of diversely functionalized 1,3-thiaselenol-2-ylmethyl selenides by a double rearrangement reaction
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-03-27 , DOI: 10.3762/bjoc.16.47 Svetlana V Amosova , Andrey A Filippov , Nataliya A Makhaeva , Alexander I Albanov , Vladimir A Potapov
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-03-27 , DOI: 10.3762/bjoc.16.47 Svetlana V Amosova , Andrey A Filippov , Nataliya A Makhaeva , Alexander I Albanov , Vladimir A Potapov
|
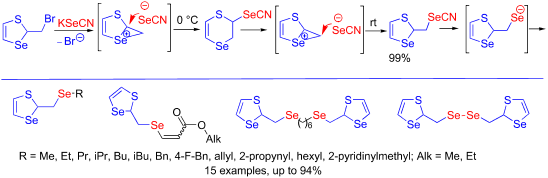
The reaction of 2-(bromomethyl)-1,3-thiaselenole with potassium selenocyanate proceeded via a rearrangement with ring expansion, leading to a six-membered 2,3-dihydro-1,4-thiaselenin-2-yl selenocyanate (kinetic product) which in turn underwent rearrangement with ring contraction to a 1,3-thiaselenol-2-ylmethyl selenocyanate (thermodynamic product). These rearrangements occurred by a nucleophilic attack of the selenocyanate anion at two different carbon atoms of the seleniranium intermediate. The efficient regioselective synthesis of alkyl, allyl, 2-propynyl, benzyl, 4-fluorobenzyl, and 2-pyridinylmethyl 1,3-thiaselenol-2-ylmethyl selenides was developed based on the generation of sodium 1,3-thiaselenol-2-ylmethylselenolate from 1,3-thiaselenol-2-ylmethyl selenocyanate or bis(1,3-thiaselenol-2-ylmethyl) diselenide followed by nucleophilic substitution reactions. Sodium 1,3-thiaselenol-2-ylmethylselenolate underwent nucleophilic addition to alkyl propiolates in a regio- and stereoselective manner affording 1,3-thiaselenol-2-ylmethyl vinyl selenides in high yields predominantly with Z-configuration. Not a single representative of the 1,3-thiaselenol-2-ylmethyl selenide scaffold has been previously described in the literature.
中文翻译:

通过双重重排反应区域和立体选择性合成功能多样的1,3-硫代噻吩酚-2-基甲基硒的新合奏
2-(溴甲基)-1,3-硫代亚硒酚与硒氰酸钾的反应是通过重排和扩环而进行的,从而导致六元2,3-二氢-1,4-硫代亚麻素-2-基硒代氰酸酯(运动产物) ),然后通过环收缩将其重排为1,3-硫代木酚-2-基甲基硒代氰酸酯(热力学产物)。这些重排是由硒氰酸酯阴离子在硒中间体的两个不同碳原子上的亲核攻击而发生的。基于1,3-硫代硒醇-2-基甲基硒醇钠的生成,开发了烷基,烯丙基,2-丙炔基,苄基,4-氟苄基和2-吡啶基甲基1,3-硫代亚麻醇-2-基甲基硒的有效区域选择性合成方法。由1,3-硫代亚麻醇-2-基甲基硒氰酸酯或bis(1,3-硫代木酚-2-基甲基)二硒化物,然后进行亲核取代反应。1,3-硫代噻吩酚-2-基甲基硒烯酸钠以区域和立体选择性方式亲核加成丙酸烷基酯,主要以高收率得到1,3-硫代硒醇-2-基甲基乙烯基硒化物Z-配置。先前在文献中没有描述过1,3-噻吩酚-2-基甲基硒化物支架的单一代表。
更新日期:2020-03-27
中文翻译:

通过双重重排反应区域和立体选择性合成功能多样的1,3-硫代噻吩酚-2-基甲基硒的新合奏
2-(溴甲基)-1,3-硫代亚硒酚与硒氰酸钾的反应是通过重排和扩环而进行的,从而导致六元2,3-二氢-1,4-硫代亚麻素-2-基硒代氰酸酯(运动产物) ),然后通过环收缩将其重排为1,3-硫代木酚-2-基甲基硒代氰酸酯(热力学产物)。这些重排是由硒氰酸酯阴离子在硒中间体的两个不同碳原子上的亲核攻击而发生的。基于1,3-硫代硒醇-2-基甲基硒醇钠的生成,开发了烷基,烯丙基,2-丙炔基,苄基,4-氟苄基和2-吡啶基甲基1,3-硫代亚麻醇-2-基甲基硒的有效区域选择性合成方法。由1,3-硫代亚麻醇-2-基甲基硒氰酸酯或bis(1,3-硫代木酚-2-基甲基)二硒化物,然后进行亲核取代反应。1,3-硫代噻吩酚-2-基甲基硒烯酸钠以区域和立体选择性方式亲核加成丙酸烷基酯,主要以高收率得到1,3-硫代硒醇-2-基甲基乙烯基硒化物Z-配置。先前在文献中没有描述过1,3-噻吩酚-2-基甲基硒化物支架的单一代表。









































 京公网安备 11010802027423号
京公网安备 11010802027423号