Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2020-03-27 , DOI: 10.1016/j.molliq.2020.112994 Jianlian Liu , Wenge Yang , Zhuo Li , Fei Ren , Hong Hao
|
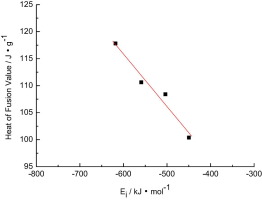
To enhance thermal stability of ionic compounds and increase thermal storage density, a series of geminal dicationic ionic compounds have been synthesized. The structures of these geminal dicationic ionic compounds were confirmed by 1H NMR and 13C NMR. Besides, thermal stability was determined by thermogravimetric analysis (TGA); melting point, heat of fusion and heat capacity were investigated by differential scanning calorimetry (DSC) for synthetic geminal dicationic ionic compounds. The effects of alkyl side-chain, the H atom at position 2 of the imidazolium ring, and anions on the thermal properties of the geminal dicationic ionic compounds were investigated. Hydrogen bonding in geminal dicationic ionic compounds was studied, in the case of C4(mim)2(Br)2, by single-crystal X-ray diffraction. The thermal analysis results indicate that geminal dicationic ionic compounds show excellent thermal stability. The decomposition temperatures of geminal dicationic ionic compounds can be up to 741.25 K, and the latent heat can reach 115.69 kJ kg−1. It is increased on average by 61.8% and 126.1%, respectively, as compared to alkyl chain ionic compounds (C4mim)Br. It can be expected that these geminal dicationic ionic compounds are suitable for thermal storage applications. Enhanced thermal storage density can thus be achieved by increasing the density and heat capacity. These values are higher than that of the monocationic ionic compounds.
It can be expected that these geminal dicationic ionic compounds are suitable for thermal storage applications.
中文翻译:

潜在热能储存的双键双键离子化合物热物理性质的实验研究
为了增强离子化合物的热稳定性并提高储热密度,已经合成了一系列双键双键离子化合物。通过1 H NMR和13 C NMR确认了这些双键双键型离子化合物的结构。另外,通过热重分析(TGA)确定热稳定性;通过差示扫描量热法(DSC)研究了合成的双基指示性离子化合物的熔点,熔融热和热容量。研究了烷基侧链,咪唑鎓环2位的H原子和阴离子对双键双键型离子化合物热性能的影响。在C 4(mim)的情况下,研究了双键双键型离子化合物中的氢键2(Br)2,通过单晶X射线衍射进行。热分析结果表明,双键双键离子化合物显示出优异的热稳定性。双键双键型离子化合物的分解温度可以高达741.25 K,潜热可以达到115.69 kJ kg -1。与烷基链离子化合物(C 4 mim)Br相比,它分别平均增加61.8%和126.1%。可以预料,这些双键双键离子化合物适用于蓄热应用。因此,可以通过增加密度和热容量来实现提高的储热密度。这些值高于单阳离子化合物的值。
可以预料,这些双键双键离子化合物适用于蓄热应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号