Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2020-03-27 , DOI: 10.1016/j.molliq.2020.113000 Xiangliang Liu , Yingjie Li , Yue Wang , Xinrui Yan , Senlin Tian , Hui Yan
|
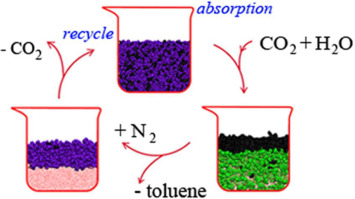
Molecular dynamics simulations were performed to investigate the absorption and desorption process of toluene by two switchable hydrophilic solvents (SHSs) named N, N-dimethylcyclohexylamine (CyNMe2) and N, N-dimethylaniline (DMA). Both CyNMe2 and DMA can absorb toluene as absorption liquid solvent, forming a homogeneous mixed oil phase. The mixture was immiscible with water, while when the SHSs were converted to their ionic forms, they would be dissolved in water, leading separation of toluene. During the desorption process of toluene, the mean square displacements show that CyNMe2H+ diffused much drastic than DMAH+. This implies that the desorption efficient of CyNMe2 is higher than DMA. Besides, when the solvents were reversed to neutral forms, the separation between CyNMe2 and water is also much quickly. By analyzing the intermolecular interactions, we found that the π-π stacking formed between aromatic rings of DMAH+ and toluene played an important role in preventing desorption of toluene. The potential of mean force (PMF) shows that, comparing with DMA, the hydration property of CyNMe2 contrasted dramatically when it was reversibly switched from being hydrophobic to hydrophilic. The study provided microscopic information on the absorption of VOCs by liquid solvents, which is expected to be helpful for designing new SHSs.
中文翻译:

分子动力学模拟研究可转换亲水性溶剂对甲苯的可逆吸收
进行分子动力学模拟以研究甲苯被两种可交换的亲水性溶剂(SHS)分别称为N,N-二甲基环己胺(CyNMe 2)和N,N-二甲基苯胺(DMA)的吸收和解吸过程。CyNMe 2和DMA均可吸收甲苯作为吸收液体溶剂,形成均匀的混合油相。该混合物与水不混溶,而当SHS转变为离子形式时,它们会溶于水,导致甲苯分离。在甲苯的解吸过程中,均方位移显示CyNMe 2 H +的扩散比DMAH +剧烈得多。这意味着CyNMe 2的解吸效率高于DMA。此外,当溶剂转变为中性形式时,CyNMe 2和水之间的分离也很快。通过分析分子间的相互作用,我们发现DMAH +的芳环与甲苯之间形成的π-π堆积在防止甲苯解吸中起重要作用。平均力(PMF)的潜力表明,与DMA相比,当CyNMe 2从疏水性转变为亲水性时,其水合性质发生了显着对比。该研究提供了有关液体溶剂吸收VOC的微观信息,这有望有助于设计新的SHS。











































 京公网安备 11010802027423号
京公网安备 11010802027423号