Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2020-03-27 , DOI: 10.1016/j.molliq.2020.112985 Qing-Wen Zhang , Chun-Chang Wang , Mei Wang , Ying-Ying Li , Xing-Yi Yue , Hai-Bo Yi , Hui-Ji Li
|
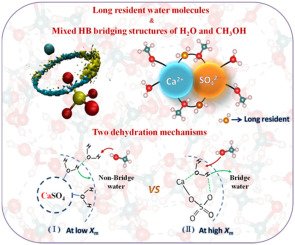
In this work, the effects of methanol on the solvation dynamics and hydrogen bond bridging structure of CaSO4 in a series of methanol-water solutions were investigated based on molecular dynamics simulations and density functional theory calculations. Results show that methanol can play important roles in regulating solvation structure and hydration dynamics in calcium sulfate solution with different ratios of methanol over water. Some water molecules in the hydrogen bond (HB) bridging structure around the Ca-OS bond of a CaSO4 pair can be replaced by methanol molecules. A HB bridging structure of double solvents can be formed even in mixed solution with high molar fraction of methanol. Due to such a HB bridging structure of dual solvents, the complete dehydration of CaSO4 ion associated structures in the mixed solvents may be quite difficult during the ion aggregation process. Our results provide useful information for understanding the phase/polymorphism selection in CaSO4 solution.
中文翻译:

甲醇对混合溶液中CaSO 4离子缔合物种的影响:溶剂动力学和氢键桥接结构
在这项工作中,基于分子动力学模拟和密度泛函理论计算,研究了甲醇对一系列甲醇-水溶液中CaSO 4的溶剂化动力学和氢键桥接结构的影响。结果表明,甲醇在不同比例的水与硫酸钙溶液中,对调节溶剂化结构和水合动力学起着重要作用。CaSO 4对的Ca-OS键周围的氢键(HB)桥接结构中的一些水分子可以被甲醇分子代替。即使在具有高摩尔分数甲醇的混合溶液中,也可以形成双溶剂的HB桥联结构。由于这种双溶剂的HB桥联结构,CaSO完全脱水在离子聚集过程中,混合溶剂中的4个离子缔合结构可能非常困难。我们的结果为理解CaSO 4溶液中的相/多态性选择提供了有用的信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号