当前位置:
X-MOL 学术
›
Appl. Clay. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Specificity and affinity of multivalent ions adsorption to kaolinite surface
Applied Clay Science ( IF 5.3 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.clay.2020.105557 Andrew Doi , Monireh Khosravi , Majid Ejtemaei , Tuan A.H. Nguyen , Anh V. Nguyen
Applied Clay Science ( IF 5.3 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.clay.2020.105557 Andrew Doi , Monireh Khosravi , Majid Ejtemaei , Tuan A.H. Nguyen , Anh V. Nguyen
|
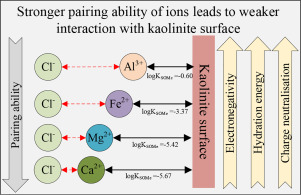
Abstract Understanding the specific adsorption mechanism(s) of multivalent ions onto clay mineral surfaces in a high salinity environment is theoretically and practically important in many areas including mineral separation and tailings disposals. This paper focuses on both qualitative and quantitative studies to describe the effect of multivalent-ion specificity on the kaolinite surface. The measured zeta potential of kaolinite particles treated with di- and trivalent chloride salts (up to 100 mM) was used to quantify the surface binding constant of metal ions to the kaolinite surface by the surface complexation model. The model results show that ion-pairing and hydration energy are two main factors that control the adsorption of di- and trivalent ions onto the kaolinite surface. The established affinity series of adsorption to kaolinite surface, Al3+ > Fe2+ > Fe3+ > Mg2+ > Ca2+, matches the hydration energy and calculated ion-pairing concentration. Further XPS analysis and molecular dynamics simulation revealed that Al3+ and Fe2+ could directly form inner-sphere complexes due to their high electronegativity and weak pairing ability with Cl− ion, while Mg2+ and Ca2+ ions adsorb onto the outer-sphere of kaolinite surface because of their strong pairing ability with Cl− ion and low electronegativity. However, higher electronegativity with large negative hydration energy leads to weaker affinity to kaolinite surface, as in the case of Fe3+. A linear relationship between surface binding constant and first hydrolysis constant of metal and kaolinite surface was also established. The outcomes of this work shed a new insight into the multivalent ion adsorption to kaolinite in controlling the selectivity and efficiency of mineral separation and dewatering processes.
中文翻译:

多价离子对高岭石表面吸附的特异性和亲和力
摘要 了解多价离子在高盐度环境中黏土矿物表面的特定吸附机制在包括矿物分离和尾矿处理在内的许多领域具有重要的理论和实践意义。本文侧重于定性和定量研究,以描述多价离子特异性对高岭石表面的影响。用二价和三价氯化物盐(高达 100 mM)处理的高岭石颗粒的测量 zeta 电位用于通过表面复合模型量化金属离子与高岭石表面的表面结合常数。模型结果表明,离子对和水合能是控制二价和三价离子吸附到高岭石表面的两个主要因素。对高岭石表面已建立的吸附亲和系列,Al3+ > Fe2+ > Fe3+ > Mg2+ > Ca2+,与水合能和计算的离子对浓度相匹配。进一步的 XPS 分析和分子动力学模拟表明,Al3+ 和 Fe2+ 具有高电负性和与 Cl− 离子的弱配对能力,可以直接形成内球配合物,而 Mg2+ 和 Ca2+ 离子则吸附在高岭石表面的外球上。与Cl-离子的配对能力强,电负性低。然而,较高的电负性和较大的负水化能导致对高岭石表面的亲和力较弱,如 Fe3+ 的情况。还建立了金属和高岭石表面的表面结合常数与第一水解常数之间的线性关系。
更新日期:2020-06-01
中文翻译:

多价离子对高岭石表面吸附的特异性和亲和力
摘要 了解多价离子在高盐度环境中黏土矿物表面的特定吸附机制在包括矿物分离和尾矿处理在内的许多领域具有重要的理论和实践意义。本文侧重于定性和定量研究,以描述多价离子特异性对高岭石表面的影响。用二价和三价氯化物盐(高达 100 mM)处理的高岭石颗粒的测量 zeta 电位用于通过表面复合模型量化金属离子与高岭石表面的表面结合常数。模型结果表明,离子对和水合能是控制二价和三价离子吸附到高岭石表面的两个主要因素。对高岭石表面已建立的吸附亲和系列,Al3+ > Fe2+ > Fe3+ > Mg2+ > Ca2+,与水合能和计算的离子对浓度相匹配。进一步的 XPS 分析和分子动力学模拟表明,Al3+ 和 Fe2+ 具有高电负性和与 Cl− 离子的弱配对能力,可以直接形成内球配合物,而 Mg2+ 和 Ca2+ 离子则吸附在高岭石表面的外球上。与Cl-离子的配对能力强,电负性低。然而,较高的电负性和较大的负水化能导致对高岭石表面的亲和力较弱,如 Fe3+ 的情况。还建立了金属和高岭石表面的表面结合常数与第一水解常数之间的线性关系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号