Energy Storage Materials ( IF 18.9 ) Pub Date : 2020-03-27 , DOI: 10.1016/j.ensm.2020.03.023 Jian Wang , Lujie Jia , Shaorong Duan , Haitao Liu , Qingbo Xiao , Tie Li , Haiyan Fan , Kun Feng , Jin Yang , Qi Wang , Meinan Liu , Jun Zhong , Wenhui Duan , Hongzhen Lin , Yuegang Zhang
|
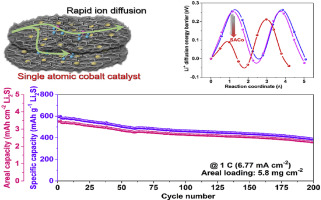
It is widely accepted that catalysts in batteries can decrease the energy barrier of conversion reactions. However, little attention has been paid to their influence on ionic diffusion in the electrode or across the interface. Herein, we report that lithium ion diffusivity can be significantly accelerated in an intrinsically electrode by atomically-distributed metal catalyst. Electrochemical measurements have found single atomic cobalt catalyst embedded in a nanocarbon network is capable of increasing the kinetics of lithium ion, providing rapid conversion reaction rate in an ultrahigh-rate Li2S battery. Meanwhile, density functional theory simulations have revealed that the lithium ion diffusion barrier on a nanocarbon surface is highly sensitive to the dopant atoms and can be decreased by introduction of cobalt atoms. The synergetic effects of the well-known Li2S decomposition catalysis and the lithium ion diffusion acceleration allow us to achieve a high Li2S mass loading cathode with superior rate performance and reversibility. The as-prepared Li2S electrode converted from cheap Li2SO4 precursors can deliver a high rate capacity (441 mA h g-1 based on Li2S at 10 C) and long lifespan for 1500 cycles at 2 C with average capacity fading of 0.04% per cycle. Impressively, the high areal mass loading cells display a specific capacity of 340 mA h g-1 based on Li2S mass at 23.4 mA cm-2 (5 C) and a long cycle life (2.26 mA h cm-2 at 6.77 mA cm-2 after 200 cycles), which have not been reported for Li2S cathodes.
中文翻译:

单原子钴催化剂可显着加速锂离子在高质量负载Li 2 S阴极中的扩散
电池中的催化剂可降低转化反应的能垒已被广泛接受。然而,很少关注它们对电极中或界面上的离子扩散的影响。在此,我们报道,通过原子分布的金属催化剂,锂离子在本征电极中的扩散率可以大大提高。电化学测量发现,嵌入纳米碳网络中的单原子钴催化剂能够提高锂离子的动力学,从而在超高速率Li 2中提供快速的转化反应速率S电池。同时,密度泛函理论模拟显示,纳米碳表面上的锂离子扩散势垒对掺杂原子高度敏感,可以通过引入钴原子来降低。众所周知的Li 2 S分解催化和锂离子扩散加速的协同作用使我们能够获得具有优异的倍率性能和可逆性的高Li 2 S质量负载阴极。由廉价的Li 2 SO 4前驱体转化而来的Li 2 S电极可提供高倍率容量(基于Li 2为441 mA h g -1S在10 C时),寿命长,在2 C时可进行1500次循环,每循环平均容量下降0.04%。令人印象深刻的是,基于23.4 mA cm -2(5 C)下的Li 2 S质量,高面积质量的称重传感器显示的比容量为340 mA h g -1,循环寿命长(在6.77 mA cm时为2.26 mA h cm -2) 200次循环后为-2),尚未报道Li 2 S阴极。











































 京公网安备 11010802027423号
京公网安备 11010802027423号