Molecular Metabolism ( IF 7.0 ) Pub Date : 2020-03-27 , DOI: 10.1016/j.molmet.2020.100984 Júlia Rodríguez-Comas 1 , Juan Moreno-Vedia 1 , Mercè Obach 1 , Carlos Castaño 2 , Sara de Pablo 1 , Gema Alcarraz-Vizán 2 , Daniela Díaz-Catalán 2 , Anna Mestre 3 , Raquel Horrillo 3 , Montserrat Costa 3 , Anna Novials 2 , Joan-Marc Servitja 2
|
Objective
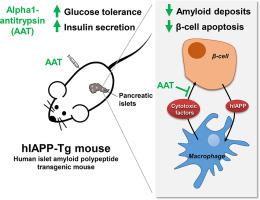
Pancreatic β-cell failure is central to the development and progression of type 2 diabetes (T2D). The aggregation of human islet amyloid polypeptide (hIAPP) has been associated with pancreatic islet inflammation and dysfunction in T2D. Alpha1-antitrypsin (AAT) is a circulating protease inhibitor with anti-inflammatory properties. Here, we sought to investigate the potential therapeutic effect of AAT treatment in a mouse model characterized by hIAPP overexpression in pancreatic β-cells.
Methods
Mice overexpressing hIAPP (hIAPP-Tg) in pancreatic β-cells were used as a model of amyloid-induced β-cell dysfunction. Glucose homeostasis was evaluated by glucose tolerance tests and insulin secretion assays. Apoptosis and amyloid formation was assessed in hIAPP-Tg mouse islets cultured at high glucose levels. Dissociated islet cells were cocultured with macrophages obtained from the peritoneal cavity.
Results
Nontreated hIAPP-Tg mice were glucose intolerant and exhibited impaired insulin secretion. Interestingly, AAT treatment improved glucose tolerance and restored the insulin secretory response to glucose in hIAPP-Tg mice. Moreover, AAT administration normalized the expression of the essential β-cell genes MafA and Pdx1, which were downregulated in pancreatic islets from hIAPP-Tg mice. AAT prevented the formation of amyloid deposits and apoptosis in hIAPP-Tg islets cultured at high glucose concentrations. Since islet macrophages mediate hIAPP-induced β-cell dysfunction, we investigated the effect of AAT in cocultures of macrophages and islet cells. AAT prevented hIAPP-induced β-cell apoptosis in these cocultures without reducing the hIAPP-induced secretion of IL-1β by macrophages. Remarkably, AAT protected β-cells against the cytotoxic effects of conditioned medium from hIAPP-treated macrophages. Similarly, AAT also abrogated the cytotoxic effects of exogenous proinflammatory cytokines on pancreatic β-cells.
Conclusions
These results demonstrate that treatment with AAT improves glucose homeostasis in mice overexpressing hIAPP and protects pancreatic β-cells from the cytotoxic actions of hIAPP mediated by macrophages. These results support the use of AAT-based therapies to recover pancreatic β-cell function for the treatment of T2D.
中文翻译:

Alpha1-抗胰蛋白酶改善了胰岛淀粉样蛋白诱导的葡萄糖耐受不良和β细胞功能障碍。
目的
胰腺β细胞衰竭是2型糖尿病(T2D)发生和发展的关键。人胰岛淀粉样多肽(hIAPP)的聚集与胰岛炎症和T2D功能障碍有关。Alpha1-抗胰蛋白酶(AAT)是一种具有抗炎特性的循环蛋白酶抑制剂。在这里,我们试图研究以小鼠β-细胞中hIAPP过表达为特征的小鼠模型中AAT治疗的潜在治疗效果。
方法
胰腺β细胞中过表达hIAPP(hIAPP-Tg)的小鼠被用作淀粉样蛋白诱导的β细胞功能障碍的模型。通过葡萄糖耐量试验和胰岛素分泌试验评估葡萄糖稳态。在高葡萄糖水平下培养的hIAPP-Tg小鼠胰岛中评估了细胞凋亡和淀粉样蛋白的形成。将解离的胰岛细胞与从腹膜腔获得的巨噬细胞共培养。
结果
未经处理的hIAPP-Tg小鼠不耐葡萄糖,并且胰岛素分泌受损。有趣的是,AAT治疗改善了hIAPP-Tg小鼠的葡萄糖耐量并恢复了胰岛素对葡萄糖的分泌反应。此外,AAT给药使必不可少的β细胞基因MafA和Pdx1的表达正常化,在来自hIAPP-Tg小鼠的胰岛中被下调。AAT阻止了在高葡萄糖浓度下培养的hIAPP-Tg胰岛中淀粉样沉积物的形成和细胞凋亡。由于胰岛巨噬细胞介导hIAPP诱导的β细胞功能障碍,我们研究了AAT在巨噬细胞和胰岛细胞共培养中的作用。AAT阻止了这些共培养物中hIAPP诱导的β细胞凋亡,而没有减少hIAPP诱导的巨噬细胞分泌IL-1β。值得注意的是,AAT保护β细胞免受hIAPP处理的巨噬细胞条件培养液的细胞毒性作用。同样,AAT也废除了外源性促炎细胞因子对胰腺β细胞的细胞毒性作用。
结论
这些结果表明,用AAT处理可改善过表达hIAPP的小鼠体内的葡萄糖稳态,并保护胰腺β细胞免受巨噬细胞介导的hIAPP的细胞毒性作用。这些结果支持使用基于AAT的疗法来恢复胰腺β细胞功能以治疗T2D。











































 京公网安备 11010802027423号
京公网安备 11010802027423号