当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
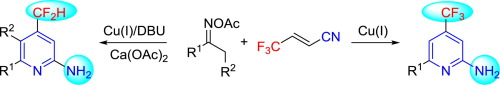
Facile Access to Fluoroalkylated 2-Aminopyridines by Cu-catalyzed [3+3] Couplings of Oxime Esters with β-CF3 Acrylonitrile
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-03-27 , DOI: 10.1016/j.tetlet.2020.151858 Xueyan Li , Yanjiang Yu , Ruobing Qi , Dachang Bai
中文翻译:

通过容易的访问到氟烷基2-氨基吡啶铜催化的[3 + 3]肟酯的联轴器与β-CF 3丙烯腈
更新日期:2020-03-27
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-03-27 , DOI: 10.1016/j.tetlet.2020.151858 Xueyan Li , Yanjiang Yu , Ruobing Qi , Dachang Bai
|
Copper catalyzed the [3+3] coupling of the oxime esters with β-CF3-acrylonitrile toward divergent fluoroalkyl-substituted 2-aminopyridines was described. This redox-neutral coupling conditions with the acrylonitrile affording the trifluoromethylated 2-aminopyridines, respectively, under the reductive conditions, difluoromethylated 2-aminopyridines were obtained. The reactions occurred under mild conditions with high functional-group compatibility and excellent regioselectivity.
中文翻译:

通过容易的访问到氟烷基2-氨基吡啶铜催化的[3 + 3]肟酯的联轴器与β-CF 3丙烯腈
铜催化的[3 + 3]的肟酯与β-CF的连接3 -丙烯腈朝向发散氟烷基取代的2-氨基吡啶进行了说明。在还原条件下,用丙烯腈分别提供三氟甲基化的2-氨基吡啶的氧化还原-中性偶联条件得到了二氟甲基化的2-氨基吡啶。反应在温和条件下进行,具有高官能团相容性和出色的区域选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号