当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactivity of some 3‐substituted‐6,8‐dimethylchromones toward some nucleophilic reagents
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-03-26 , DOI: 10.1002/jhet.3975 Al‐Shimaa Badran 1 , Nasser M. El‐Gohary 1 , Magdy A. Ibrahim 1 , Salsabeel H. Hashiem 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-03-26 , DOI: 10.1002/jhet.3975 Al‐Shimaa Badran 1 , Nasser M. El‐Gohary 1 , Magdy A. Ibrahim 1 , Salsabeel H. Hashiem 1
Affiliation
|
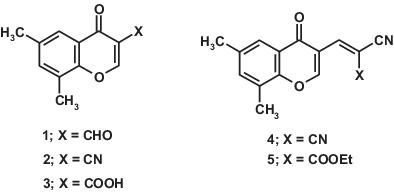
The reactivity of 3‐substituted‐6,8‐dimethylchromone derivatives 1 ‐5 was investigated toward selected nucleophilic reagents namely; 3‐amino‐1,2,4‐triazole, 2‐aminobenzimidazole, 7‐chloro‐4‐hydrazinoquinoline, and 5,6‐diphenyl‐3‐hydrazino‐1,2,4‐triazine. These nucleophiles were allowed to react with 6,8‐dimethylchromone‐3‐carboxaldehyde (1 ) through condensation with the aldehyde group with opening of γ‐pyrone ring giving compounds 6 , 7 , 10, and 11 . Reactions with 6,8‐dimethylchromone‐3‐carbonitrile (2 ), and 6,8‐dimethylchromone‐3‐carboxylic acid (3 ) occur via attack at position 2 in both compounds followed by cycloaddition onto the nitrile function (in case of carbonitrile 2 ) or decarboxylation and cyclocondensation with the carbonyl group (in case of carboxylic acid 3 ). The current nucleophilic reagents reacted with simple condensation products 4 and 5 , in boiling dioxane, through nucleophilic addition at the exocyclic vinyl bond followed by addition at the CN group giving 6,8‐dimethylchromone linked various heterocyclic systems 20 ‐27 .
中文翻译:

某些3-取代6,8-二甲基色酮对某些亲核试剂的反应性
3-取代的-6,8- dimethylchromone衍生物的反应性1 - 5被朝向即选择亲核试剂研究; 3-氨基1,2,4-三唑,2-氨基苯并咪唑,7-氯-4-肼基喹啉和5,6-二苯基-3-肼基1,2,4-三嗪。这些亲核试剂被允许与6,8- dimethylchromone -3-甲醛(反应1)通过缩合与醛基与γ吡喃酮环得到的化合物的开口6,7,10,和11。与6,8-二甲基色酮-3-腈(2)和6,8-二甲基色酮-3-羧酸(3)的发生是通过两种化合物在位置2上的进攻,然后在腈官能团上进行环加成(对于腈2而言),或与羰基进行脱羧和环缩合(对于羧酸3而言)。当前亲核试剂用简单的缩合产物反应4和5,在沸腾的二恶烷,通过亲核加成在环外的乙烯基键,随后加入在CN组给予6,8- dimethylchromone连接各种杂环系统20 - 27。
更新日期:2020-03-26
中文翻译:

某些3-取代6,8-二甲基色酮对某些亲核试剂的反应性
3-取代的-6,8- dimethylchromone衍生物的反应性1 - 5被朝向即选择亲核试剂研究; 3-氨基1,2,4-三唑,2-氨基苯并咪唑,7-氯-4-肼基喹啉和5,6-二苯基-3-肼基1,2,4-三嗪。这些亲核试剂被允许与6,8- dimethylchromone -3-甲醛(反应1)通过缩合与醛基与γ吡喃酮环得到的化合物的开口6,7,10,和11。与6,8-二甲基色酮-3-腈(2)和6,8-二甲基色酮-3-羧酸(3)的发生是通过两种化合物在位置2上的进攻,然后在腈官能团上进行环加成(对于腈2而言),或与羰基进行脱羧和环缩合(对于羧酸3而言)。当前亲核试剂用简单的缩合产物反应4和5,在沸腾的二恶烷,通过亲核加成在环外的乙烯基键,随后加入在CN组给予6,8- dimethylchromone连接各种杂环系统20 - 27。











































 京公网安备 11010802027423号
京公网安备 11010802027423号