当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and synthesis of novel quinoline derivatives bearing oxadiazole, isoxazoline, triazolothiadiazole, triazolothiadiazine, and piperazine moieties
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-03-26 , DOI: 10.1002/jhet.3907 Zhiren Tang 1 , Yang Peng 1 , Fangming Liu 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-03-26 , DOI: 10.1002/jhet.3907 Zhiren Tang 1 , Yang Peng 1 , Fangming Liu 1
Affiliation
|
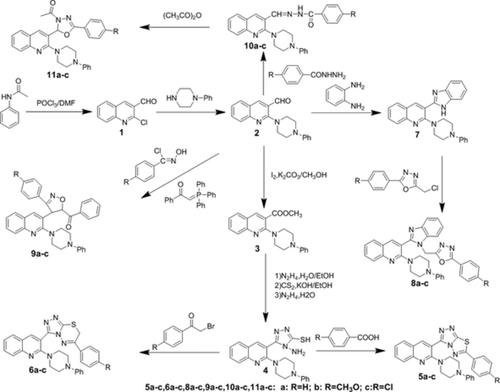
A new series of 2,3‐disubstituted quinoline derivatives were synthesized from 2‐chloroquinoline‐3‐carbaldehyde. In the reaction sequence, acetanilide was cyclized to give 2‐chloroquinoline‐3‐carbaldehyde 1 , which was transformed to 2‐(4‐phenylpiperazin‐1‐yl)quinolin‐3‐carbaldehyde 2 by reaction with 4‐phenylpiperazine in DMF‐containing anhydrous K2CO3; then, compound 2 was oxidized by iodine in methanol, and methyl 2‐(4‐phenylpiperazin‐1‐yl)quinoline‐3‐carboxylate 3 was synthesized. The key intermediate 4 , 4‐amino‐5‐[2‐(4‐phenylpiperazin‐1‐yl)quinolin‐3‐yl]‐4H ‐1,2,4‐triazole‐3‐thiol, was prepared using the ester 3 by a series of step. Reaction of 5 with various aromatic carboxylic acids or phenacyl bromides yielded 1,2,4‐triazolo[3,4‐b ][1,3,4]thiadiazoles 5a‐c and 1,2,4‐triazolo[3,4‐b ][1,3,4]thiadiazines 6a‐c , respectively. Moreover, compound 2 condensed with o ‐phenylenediamine to give 2‐[2‐(4‐phenylpiperazin‐1‐yl)quinolin‐3‐yl]‐1H ‐benzimidazole 7 . Interaction of 7 and 2‐chloromethyl‐5‐aryl‐1,3,4‐oxadiazoles in the presence of K2CO3 led to the title compounds 8a‐c . Furthermore, 4,5‐dihydroisoxazoline derivatives 9a‐c were obtained by the reaction of readily accessible starting materials including 2‐(4‐phenylpiperazin‐1‐yl)quinolin‐3‐carbaldehyde 2 , 1‐phenyl‐2‐(triphenylphosphoranylidene)ethanone and hydroximoyl chlorides under mild conditions in the presence of Et3N. The hydrazone intermediates 10a‐c were obtained by the condensation of 2 with aroylhydrazides in ethanol, then, refluxing in acetic anhydride yielded 3‐acetyl‐5‐aryl‐2‐[2‐(4‐phenylpiperazin‐1‐yl)quinolin‐3‐yl]‐2,3‐dihydro‐1,3,4‐oxadiazoles 11a‐c . Structures of these compounds were established by their elemental analysis, IR, 1H NMR, and mass spectral data.
中文翻译:

设计和合成带有恶二唑,异恶唑啉,三唑并噻二唑,三唑并噻二嗪和哌嗪部分的新型喹啉衍生物
从2-氯喹啉-3-甲醛中合成了一系列新的2,3-二取代喹啉衍生物。在反应顺序中,对乙酰苯胺进行环化以生成2-氯喹啉3-甲醛1,通过与4-苯基哌嗪在含DMF的反应将其转化为2-(4-苯基哌嗪-1-基)喹啉3-甲醛2。无水K 2 CO 3 ; 然后,化合物2在甲醇中被碘氧化,合成了2-(4-苯基哌嗪-1-基)喹啉-3-羧酸甲酯3。关键中间体4,4-氨基-5- [2-(4-苯基哌嗪-1-基)喹啉-3-基] -4- ħ -1,2,4-三唑-3-硫醇,用的是酯制备3通过一系列步骤。的反应5与各种芳族羧酸或苯甲酰甲基溴化物,得到1,2,4-三唑并[3,4- b ] [1,3,4]噻二唑5A-C和1,2,4-三唑并[3,4 b分别为[1,3,4]噻二嗪6a-c。此外,化合物2与邻苯二胺缩合得到2- [2-(4-苯基哌嗪-1-基)喹啉-3-基] -1 H-苯并咪唑7。在K 2 CO 3存在下7和2-氯甲基-5-芳基-1,3,4-恶二唑的相互作用产生标题化合物8a-c。此外,4,5- dihydroisoxazoline衍生物9A-C是由容易获得的起始材料的包括2-(4-苯基哌嗪-1-基)喹啉-3-甲醛的反应获得的2,1-苯基-2-(三苯基亚正膦)乙酮并在的Et存在下在温和条件下肟基氯化物3的腙中间体N. 10a到10c被的缩合得到2与乙醇aroylhydrazides,然后,在乙酸酐中回流,得到3-乙酰基-5-芳基- 2- [ 2-(4-苯基哌嗪-1-基)喹啉-3-基] -2,3-二氢-1,3,4-恶二唑11a-c。这些化合物的结构通过其元素分析IR 1来确定。1 H NMR和质谱数据。
更新日期:2020-03-26
中文翻译:

设计和合成带有恶二唑,异恶唑啉,三唑并噻二唑,三唑并噻二嗪和哌嗪部分的新型喹啉衍生物
从2-氯喹啉-3-甲醛中合成了一系列新的2,3-二取代喹啉衍生物。在反应顺序中,对乙酰苯胺进行环化以生成2-氯喹啉3-甲醛1,通过与4-苯基哌嗪在含DMF的反应将其转化为2-(4-苯基哌嗪-1-基)喹啉3-甲醛2。无水K 2 CO 3 ; 然后,化合物2在甲醇中被碘氧化,合成了2-(4-苯基哌嗪-1-基)喹啉-3-羧酸甲酯3。关键中间体4,4-氨基-5- [2-(4-苯基哌嗪-1-基)喹啉-3-基] -4- ħ -1,2,4-三唑-3-硫醇,用的是酯制备3通过一系列步骤。的反应5与各种芳族羧酸或苯甲酰甲基溴化物,得到1,2,4-三唑并[3,4- b ] [1,3,4]噻二唑5A-C和1,2,4-三唑并[3,4 b分别为[1,3,4]噻二嗪6a-c。此外,化合物2与邻苯二胺缩合得到2- [2-(4-苯基哌嗪-1-基)喹啉-3-基] -1 H-苯并咪唑7。在K 2 CO 3存在下7和2-氯甲基-5-芳基-1,3,4-恶二唑的相互作用产生标题化合物8a-c。此外,4,5- dihydroisoxazoline衍生物9A-C是由容易获得的起始材料的包括2-(4-苯基哌嗪-1-基)喹啉-3-甲醛的反应获得的2,1-苯基-2-(三苯基亚正膦)乙酮并在的Et存在下在温和条件下肟基氯化物3的腙中间体N. 10a到10c被的缩合得到2与乙醇aroylhydrazides,然后,在乙酸酐中回流,得到3-乙酰基-5-芳基- 2- [ 2-(4-苯基哌嗪-1-基)喹啉-3-基] -2,3-二氢-1,3,4-恶二唑11a-c。这些化合物的结构通过其元素分析IR 1来确定。1 H NMR和质谱数据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号