当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ruthenium-Catalyzed Site-Selective Trifluoromethylations and (Per)Fluoroalkylations of Anilines and Indoles.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-03-26 , DOI: 10.1002/chem.202001439 Yang Li 1, 2 , Helfried Neumann 1 , Matthias Beller 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-03-26 , DOI: 10.1002/chem.202001439 Yang Li 1, 2 , Helfried Neumann 1 , Matthias Beller 1
Affiliation
|
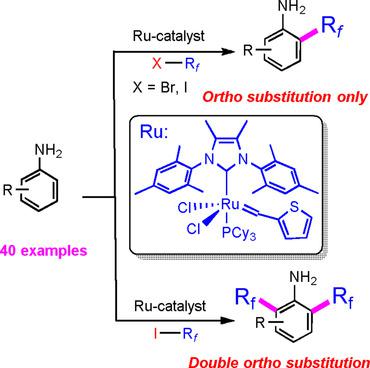
Introducing (per)fluoroalkyl groups into arenes continues to be an interesting, but challenging area in organofluorine chemistry. We herein report an ortho-selective C-H perfluoroalkylation including trifluoromethylations of anilines and indoles without the need of protecting groups using Rf I and Rf Br as commercially available reagents. The availability and price of the starting materials and the inherent selectivity make this novel methodology attractive for the synthesis of diverse (per)fluoroalkylated building blocks, for example, for bioactive compounds and materials.
中文翻译:

钌催化的苯胺和吲哚的位点选择性三氟甲基化和(全)氟烷基化。
将(全)氟烷基引入芳烃中仍然是有机氟化学中一个有趣但具有挑战性的领域。我们在此报道了使用 Rf I 和 Rf Br 作为市售试剂的邻位选择性 CH 全氟烷基化,包括苯胺和吲哚的三氟甲基化,无需保护基团。起始材料的可用性和价格以及固有的选择性使得这种新颖的方法对于合成各种(全)氟烷基化结构单元(例如生物活性化合物和材料)具有吸引力。
更新日期:2020-03-26
中文翻译:

钌催化的苯胺和吲哚的位点选择性三氟甲基化和(全)氟烷基化。
将(全)氟烷基引入芳烃中仍然是有机氟化学中一个有趣但具有挑战性的领域。我们在此报道了使用 Rf I 和 Rf Br 作为市售试剂的邻位选择性 CH 全氟烷基化,包括苯胺和吲哚的三氟甲基化,无需保护基团。起始材料的可用性和价格以及固有的选择性使得这种新颖的方法对于合成各种(全)氟烷基化结构单元(例如生物活性化合物和材料)具有吸引力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号