当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective 1,3-Dipolar [6+4] Cycloaddition of Pyrylium Ions and Fulvenes towards Cyclooctanoids.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-03-26 , DOI: 10.1002/chem.202001369 David McLeod 1 , Alessio Cherubini-Celli 1 , Nisanhi Sivasothirajah 1 , Christina H McCulley 1 , Mette Louise Christensen 1 , Karl Anker Jørgensen 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-03-26 , DOI: 10.1002/chem.202001369 David McLeod 1 , Alessio Cherubini-Celli 1 , Nisanhi Sivasothirajah 1 , Christina H McCulley 1 , Mette Louise Christensen 1 , Karl Anker Jørgensen 1
Affiliation
|
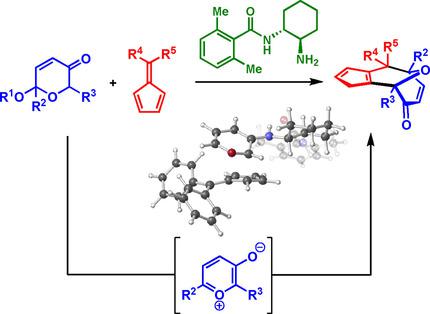
Organocatalytic enantioselective 1,3‐dipolar [6+4] cycloadditions of pyrylium ion intermediates with fulvenes promoted by a chiral primary amine catalyst have been developed to proceed in moderate to good yields and high enantioselectivities. The resultant chiral bicyclo[6.3.0]undecane scaffold containing a transannular bridging ether is densely functionalised providing a rigid scaffold for further manipulations. Computational studies give important insights into the role of the primary amine catalyst. Analysis of the reaction shows that the catalytic reaction proceeds in a step‐wise manner and rationalises the stereochemical outcome of the reaction. Several stereoselective complexity‐generating transformations, facilitated by the diverse functional groups and transannular bridge, are presented, highlighting the versatility of the core towards a number of the cyclooctanoid natural products.
中文翻译:

吡咯离子和丁二烯向环庚烷的对映选择性1,3-偶极[6 + 4]环加成。
吡啶离子中间体的有机催化对映选择性1,3-偶极[6 + 4]环加成与手性伯胺催化剂促进的富烯的开发已以中等至良好的收率和高对映选择性进行。所得到的含有跨环桥联醚的手性双环[6.3.0]十一烷骨架被密集地官能化,从而提供了一种刚性骨架,以便进一步操作。计算研究为伯胺催化剂的作用提供了重要的见识。反应分析表明,催化反应是逐步进行的,并合理化了反应的立体化学结果。提出了由不同的功能组和跨环桥促成的几种立体选择性生成复杂度的转换,
更新日期:2020-03-26
中文翻译:

吡咯离子和丁二烯向环庚烷的对映选择性1,3-偶极[6 + 4]环加成。
吡啶离子中间体的有机催化对映选择性1,3-偶极[6 + 4]环加成与手性伯胺催化剂促进的富烯的开发已以中等至良好的收率和高对映选择性进行。所得到的含有跨环桥联醚的手性双环[6.3.0]十一烷骨架被密集地官能化,从而提供了一种刚性骨架,以便进一步操作。计算研究为伯胺催化剂的作用提供了重要的见识。反应分析表明,催化反应是逐步进行的,并合理化了反应的立体化学结果。提出了由不同的功能组和跨环桥促成的几种立体选择性生成复杂度的转换,











































 京公网安备 11010802027423号
京公网安备 11010802027423号