当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unique β-Turn Peptoid Structures and Their Application as Asymmetric Catalysts.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-03-26 , DOI: 10.1002/chem.202000595 Chandra Mohan Darapaneni 1 , Pritam Ghosh 1 , Totan Ghosh 1 , Galia Maayan 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-03-26 , DOI: 10.1002/chem.202000595 Chandra Mohan Darapaneni 1 , Pritam Ghosh 1 , Totan Ghosh 1 , Galia Maayan 1
Affiliation
|
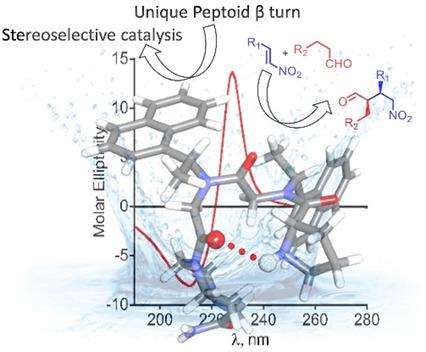
Peptoids, N‐substituted glycine oligomers, represent an important class of peptidomimetics that can fold into three‐dimensional structures in solution. Most of the folded peptoid structures, however, resemble helices, and this can limit their applications, specifically in asymmetric catalysis. In this work, for the first time, unique examples of pyrrolidine‐based β‐turn‐like peptoids are described and characterized, both in the solid state, by single‐crystal X‐ray analysis, and in solution, by circular dichroism spectroscopy. Furthermore, their highly efficient and enantioselective catalytic activity for the production of γ‐nitro aldehydes by asymmetric Michael reaction in water was demonstrated. The structural properties and DFT‐D3 calculations of the new β‐turn‐like peptoids, as well as catalytic and spectroscopic studies on designed pyrrolidine‐based helical peptoids, suggest that the β‐turn structure plays a key role in the stereoselectivity of the catalytic reaction.
中文翻译:

独特的β-Turn类肽结构及其作为不对称催化剂的应用。
类肽,N-取代的甘氨酸低聚物,代表了一类重要的拟肽,可以在溶液中折叠成三维结构。然而,大多数折叠的类肽结构类似于螺旋,这可能限制了它们的应用,特别是在不对称催化中。在这项工作中,首次描述了吡咯烷类β-turn类肽的独特实例,并在固态,单晶X射线分析和溶液中通过圆二色光谱进行了表征。此外,还证明了它们通过在水中的不对称迈克尔反应产生γ-硝基醛的高效和对映选择性催化活性。新的β-turn-like类肽的结构性质和DFT-D3计算,
更新日期:2020-03-26
中文翻译:

独特的β-Turn类肽结构及其作为不对称催化剂的应用。
类肽,N-取代的甘氨酸低聚物,代表了一类重要的拟肽,可以在溶液中折叠成三维结构。然而,大多数折叠的类肽结构类似于螺旋,这可能限制了它们的应用,特别是在不对称催化中。在这项工作中,首次描述了吡咯烷类β-turn类肽的独特实例,并在固态,单晶X射线分析和溶液中通过圆二色光谱进行了表征。此外,还证明了它们通过在水中的不对称迈克尔反应产生γ-硝基醛的高效和对映选择性催化活性。新的β-turn-like类肽的结构性质和DFT-D3计算,











































 京公网安备 11010802027423号
京公网安备 11010802027423号