当前位置:
X-MOL 学术
›
Signal Transduct. Target Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The circFASN/miR-33a pathway participates in tacrolimus-induced dysregulation of hepatic triglyceride homeostasis.
Signal Transduction and Targeted Therapy ( IF 40.8 ) Pub Date : 2020-03-27 , DOI: 10.1038/s41392-020-0105-2 Chenzhi Zhang 1, 2 , Kangchen Chen 1, 2 , Rongli Wei 1, 2 , Guanghan Fan 1, 2 , Xuechun Cai 1, 2 , Li Xu 1, 2 , Beini Cen 2 , Jianguo Wang 1, 2 , Haiyang Xie 2 , Shusen Zheng 1, 2, 3 , Xiao Xu 1, 2
Signal Transduction and Targeted Therapy ( IF 40.8 ) Pub Date : 2020-03-27 , DOI: 10.1038/s41392-020-0105-2 Chenzhi Zhang 1, 2 , Kangchen Chen 1, 2 , Rongli Wei 1, 2 , Guanghan Fan 1, 2 , Xuechun Cai 1, 2 , Li Xu 1, 2 , Beini Cen 2 , Jianguo Wang 1, 2 , Haiyang Xie 2 , Shusen Zheng 1, 2, 3 , Xiao Xu 1, 2
Affiliation
|
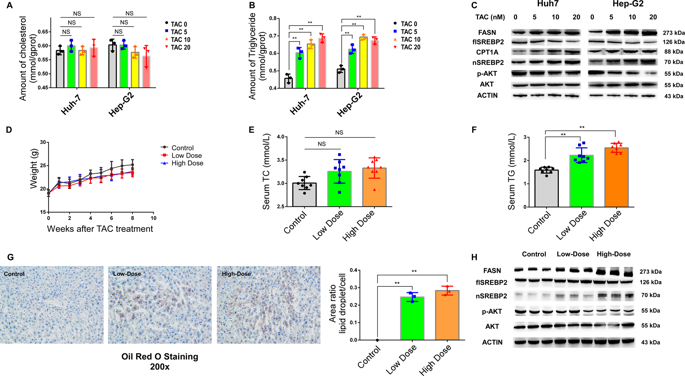
Dyslipidemia exhibits a high incidence after liver transplantation, in which tacrolimus, a widely used immunosuppressant, plays a fundamental role. MicroRNAs and related circRNAs represent a class of noncoding RNAs that have been recognized as important regulators of genes associated with lipid metabolism. However, their transcriptional activities and functional mechanisms in tacrolimus-related dyslipidemia remain unclear. In this study, we observed that tacrolimus could induce triglyceride accumulation in hepatocytes by stimulating sterol response element-binding proteins (SREBPs) and miR-33a. Our in silico and experimental analyses identified miR-33a as a direct target of circFASN. Tacrolimus could downregulate circFASN and result in elevated miR-33a in vivo and in vitro. Overexpression of circFASN or silencing of miR-33a decreased the promoting effects of tacrolimus on triglyceride accumulation. Clinically, the incidence of dyslipidemia in liver transplant recipients with elevated serum miR-33a after liver transplantation was higher than that in patients without elevated serum miR-33a (46.3% vs. 18.8% p = 0.012, n = 73). Our results showed that the circFASN/miR-33a regulatory system plays a distinct role in tacrolimus-induced disruption of lipid homeostasis. MiR-33a is likely a risk factor for tacrolimus-related dyslipidemia, providing a potential therapeutic target to combat tacrolimus-induced dyslipidemia after liver transplantation.
中文翻译:

circFASN/miR-33a 通路参与他克莫司诱导的肝脏甘油三酯稳态失调。
肝移植后血脂异常的发生率很高,其中他克莫司作为一种广泛使用的免疫抑制剂,起着重要的作用。MicroRNAs 和相关的 circRNAs 代表了一类非编码 RNAs,它们被认为是与脂质代谢相关的基因的重要调节因子。然而,它们在他克莫司相关血脂异常中的转录活性和功能机制尚不清楚。在这项研究中,我们观察到他克莫司可以通过刺激甾醇反应元件结合蛋白 (SREBPs) 和 miR-33a 来诱导肝细胞中甘油三酯的积累。我们的计算机和实验分析将 miR-33a 确定为 circFASN 的直接目标。他克莫司可以下调 circFASN 并导致体内和体外 miR-33a 升高。circFASN的过表达或miR-33a的沉默降低了他克莫司对甘油三酯积累的促进作用。临床上,肝移植后血清 miR-33a 升高的肝移植受者血脂异常发生率高于血清 miR-33a 未升高的患者(46.3% vs. 18.8% p = 0.012,n = 73)。我们的结果表明,circFASN/miR-33a 调节系统在他克莫司诱导的脂质稳态破坏中发挥着独特的作用。MiR-33a 可能是他克莫司相关血脂异常的危险因素,为对抗肝移植后他克莫司引起的血脂异常提供了潜在的治疗靶点。肝移植后血清miR-33a升高的肝移植受者血脂异常发生率高于血清miR-33a未升高的患者(46.3% vs. 18.8% p = 0.012, n = 73)。我们的结果表明,circFASN/miR-33a 调节系统在他克莫司诱导的脂质稳态破坏中发挥着独特的作用。MiR-33a 可能是他克莫司相关血脂异常的危险因素,为对抗肝移植后他克莫司引起的血脂异常提供了潜在的治疗靶点。肝移植后血清miR-33a升高的肝移植受者血脂异常发生率高于血清miR-33a未升高的患者(46.3% vs. 18.8% p = 0.012, n = 73)。我们的结果表明,circFASN/miR-33a 调节系统在他克莫司诱导的脂质稳态破坏中发挥着独特的作用。MiR-33a 可能是他克莫司相关血脂异常的危险因素,为对抗肝移植后他克莫司引起的血脂异常提供了潜在的治疗靶点。
更新日期:2020-03-27
中文翻译:

circFASN/miR-33a 通路参与他克莫司诱导的肝脏甘油三酯稳态失调。
肝移植后血脂异常的发生率很高,其中他克莫司作为一种广泛使用的免疫抑制剂,起着重要的作用。MicroRNAs 和相关的 circRNAs 代表了一类非编码 RNAs,它们被认为是与脂质代谢相关的基因的重要调节因子。然而,它们在他克莫司相关血脂异常中的转录活性和功能机制尚不清楚。在这项研究中,我们观察到他克莫司可以通过刺激甾醇反应元件结合蛋白 (SREBPs) 和 miR-33a 来诱导肝细胞中甘油三酯的积累。我们的计算机和实验分析将 miR-33a 确定为 circFASN 的直接目标。他克莫司可以下调 circFASN 并导致体内和体外 miR-33a 升高。circFASN的过表达或miR-33a的沉默降低了他克莫司对甘油三酯积累的促进作用。临床上,肝移植后血清 miR-33a 升高的肝移植受者血脂异常发生率高于血清 miR-33a 未升高的患者(46.3% vs. 18.8% p = 0.012,n = 73)。我们的结果表明,circFASN/miR-33a 调节系统在他克莫司诱导的脂质稳态破坏中发挥着独特的作用。MiR-33a 可能是他克莫司相关血脂异常的危险因素,为对抗肝移植后他克莫司引起的血脂异常提供了潜在的治疗靶点。肝移植后血清miR-33a升高的肝移植受者血脂异常发生率高于血清miR-33a未升高的患者(46.3% vs. 18.8% p = 0.012, n = 73)。我们的结果表明,circFASN/miR-33a 调节系统在他克莫司诱导的脂质稳态破坏中发挥着独特的作用。MiR-33a 可能是他克莫司相关血脂异常的危险因素,为对抗肝移植后他克莫司引起的血脂异常提供了潜在的治疗靶点。肝移植后血清miR-33a升高的肝移植受者血脂异常发生率高于血清miR-33a未升高的患者(46.3% vs. 18.8% p = 0.012, n = 73)。我们的结果表明,circFASN/miR-33a 调节系统在他克莫司诱导的脂质稳态破坏中发挥着独特的作用。MiR-33a 可能是他克莫司相关血脂异常的危险因素,为对抗肝移植后他克莫司引起的血脂异常提供了潜在的治疗靶点。









































 京公网安备 11010802027423号
京公网安备 11010802027423号