当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring the scope of DBU-promoted amidations of 7-methoxycarbonylpterin
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-03-26 , DOI: 10.3762/bjoc.16.46 Anna R Bockman , Jeffrey M Pruet
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-03-26 , DOI: 10.3762/bjoc.16.46 Anna R Bockman , Jeffrey M Pruet
|
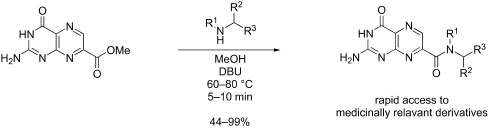
The synthetic utility of pterins is often hampered by the notorious insolubility of this heterocycle, slowing the development of medicinally relevant pteridine derivatives. Reactions which expedite the development of new pterins are thus of great importance. Through a dual role of diazabicycloundecene (DBU), 7-carboxymethylpterin is converted to the soluble DBU salt, with additional DBU promoting an ester-to-amide transformation. We have explored this reaction to assess its scope and identify structural features in the amines which significantly affect success, monitored the reaction kinetics using a pseudo-first order kinetics model, and further adapted the reaction conditions to allow for product formation in as little as 5 min, with yields often >80%.
中文翻译:

探索DBU促进7-甲氧羰基蝶呤酰胺化的范围
蝶呤的合成效用常常受到该杂环臭名昭著的不溶性的阻碍,从而减缓了与医学相关的蝶啶衍生物的开发。因此,加速新蝶呤开发的反应非常重要。通过二氮杂双环十一碳烯(DBU)的双重作用,将7-羧甲基蝶呤转化为可溶性DBU盐,另外的DBU促进了酯到酰胺的转化。我们已经研究了该反应以评估其范围并确定对胺有重大影响的结构特征,使用拟一级动力学模型监控了反应动力学,并进一步调整了反应条件以使产物形成少至5分钟,收率通常> 80%。
更新日期:2020-03-27
中文翻译:

探索DBU促进7-甲氧羰基蝶呤酰胺化的范围
蝶呤的合成效用常常受到该杂环臭名昭著的不溶性的阻碍,从而减缓了与医学相关的蝶啶衍生物的开发。因此,加速新蝶呤开发的反应非常重要。通过二氮杂双环十一碳烯(DBU)的双重作用,将7-羧甲基蝶呤转化为可溶性DBU盐,另外的DBU促进了酯到酰胺的转化。我们已经研究了该反应以评估其范围并确定对胺有重大影响的结构特征,使用拟一级动力学模型监控了反应动力学,并进一步调整了反应条件以使产物形成少至5分钟,收率通常> 80%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号