当前位置:
X-MOL 学术
›
Sci. Total Environ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of Fe(II) on Sb(III) oxidation and adsorption by MnO2 under acidic conditions.
Science of the Total Environment ( IF 8.2 ) Pub Date : 2020-03-26 , DOI: 10.1016/j.scitotenv.2020.138209 Chengjun Zhang 1 , Mengchang He 1 , Wei Ouyang 1 , Chunye Lin 1 , Xitao Liu 1
Science of the Total Environment ( IF 8.2 ) Pub Date : 2020-03-26 , DOI: 10.1016/j.scitotenv.2020.138209 Chengjun Zhang 1 , Mengchang He 1 , Wei Ouyang 1 , Chunye Lin 1 , Xitao Liu 1
Affiliation
|
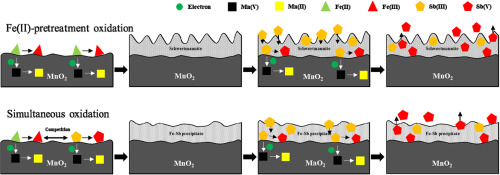
The transformation and transport of Sb are significantly influenced by strong oxides (e.g. MnO2) in the natural environment. Furthermore, Fe(II) can coexist with Sb(III) and MnO2 in waters contaminated by acidic mine drainage. However, role of Fe(II) in Sb(III) oxidation and adsorption by MnO2 remains unclear. Therefore, in the present study, the effects of Fe(II) on the oxidation and adsorption of Sb(III) by MnO2 under acidic conditions (pH 3) and the mechanism thereof were comprehensively investigated. The results of kinetic experiments showed that, in the presence of soluble Fe(II), Sb(III) oxidation is inhibited, but adsorption is promoted. Further characterization confirmed that Fe(III) compounds are formed around MnO2 particles and that these inhibit Sb(III) oxidation. However, two different Fe(III) compounds are formed around MnO2 particles depending on how the Fe(II) is introduced into the experimental system. In the simultaneous oxidation system, poorly crystallized or amorphous FeSb precipitates are formed (probably FeSbO4) around MnO2 particles, while in the Fe(II) pretreated oxidation system, schwertmannite is formed. Thus, the present study revealed that Fe(II) is critical to Sb(III) oxidation and adsorption by MnO2 and that the mechanism of its action is depend upon how it is introduced into the reaction system. This information is of relevance to predicting the fate of Sb.
中文翻译:

Fe(II)对Sb(III)在酸性条件下氧化和MnO2吸附的影响。
在自然环境中,Sb的转化和迁移受到强氧化物(例如MnO2)的显着影响。此外,Fe(II)可以与Sb(III)和MnO2共存于被酸性矿井排水污染的水中。但是,Fe(II)在Sb(III)氧化和MnO2吸附中的作用仍不清楚。因此,在本研究中,全面研究了Fe(II)对MnO2在酸性条件(pH 3)下氧化和吸附Sb(III)的影响及其机理。动力学实验结果表明,在可溶性Fe(II)的存在下,Sb(III)的氧化被抑制,但吸附被促进。进一步表征证实,Fe(III)化合物在MnO2颗粒周围形成,并且它们抑制Sb(III)的氧化。然而,根据将Fe(II)引入实验系统的方式,在MnO2颗粒周围会形成两种不同的Fe(III)化合物。在同时氧化系统中,在MnO2颗粒周围形成结晶不良或无定形的FeSb沉淀物(可能是FeSbO4),而在经过Fe(II)预处理的氧化系统中,形成了Schwertmannite。因此,本研究表明Fe(II)对于MnO2氧化和吸附Sb(III)至关重要,其作用机理取决于将其引入反应体系的方式。此信息与预测Sb的命运有关。而在Fe(II)预处理的氧化系统中,形成了Schwertmannite。因此,本研究表明,Fe(II)对于MnO2氧化和吸附Sb(III)至关重要,其作用机理取决于将其引入反应体系的方式。此信息与预测Sb的命运有关。而在Fe(II)预处理的氧化系统中,形成了Schwertmannite。因此,本研究表明Fe(II)对于MnO2氧化和吸附Sb(III)至关重要,其作用机理取决于将其引入反应体系的方式。此信息与预测Sb的命运有关。
更新日期:2020-03-27
中文翻译:

Fe(II)对Sb(III)在酸性条件下氧化和MnO2吸附的影响。
在自然环境中,Sb的转化和迁移受到强氧化物(例如MnO2)的显着影响。此外,Fe(II)可以与Sb(III)和MnO2共存于被酸性矿井排水污染的水中。但是,Fe(II)在Sb(III)氧化和MnO2吸附中的作用仍不清楚。因此,在本研究中,全面研究了Fe(II)对MnO2在酸性条件(pH 3)下氧化和吸附Sb(III)的影响及其机理。动力学实验结果表明,在可溶性Fe(II)的存在下,Sb(III)的氧化被抑制,但吸附被促进。进一步表征证实,Fe(III)化合物在MnO2颗粒周围形成,并且它们抑制Sb(III)的氧化。然而,根据将Fe(II)引入实验系统的方式,在MnO2颗粒周围会形成两种不同的Fe(III)化合物。在同时氧化系统中,在MnO2颗粒周围形成结晶不良或无定形的FeSb沉淀物(可能是FeSbO4),而在经过Fe(II)预处理的氧化系统中,形成了Schwertmannite。因此,本研究表明Fe(II)对于MnO2氧化和吸附Sb(III)至关重要,其作用机理取决于将其引入反应体系的方式。此信息与预测Sb的命运有关。而在Fe(II)预处理的氧化系统中,形成了Schwertmannite。因此,本研究表明,Fe(II)对于MnO2氧化和吸附Sb(III)至关重要,其作用机理取决于将其引入反应体系的方式。此信息与预测Sb的命运有关。而在Fe(II)预处理的氧化系统中,形成了Schwertmannite。因此,本研究表明Fe(II)对于MnO2氧化和吸附Sb(III)至关重要,其作用机理取决于将其引入反应体系的方式。此信息与预测Sb的命运有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号