当前位置:
X-MOL 学术
›
Spectrochim. Acta. A Mol. Biomol. Spectrosc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New spectrofluorimetric analysis of empagliflozin in its tablets and human plasma using two level full factorial design.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2020-03-26 , DOI: 10.1016/j.saa.2020.118307 Mahmoud A Omar 1 , Hytham M Ahmed 2 , Hany A Batakoushy 2 , Mohamed A Abdel Hamid 3
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2020-03-26 , DOI: 10.1016/j.saa.2020.118307 Mahmoud A Omar 1 , Hytham M Ahmed 2 , Hany A Batakoushy 2 , Mohamed A Abdel Hamid 3
Affiliation
|
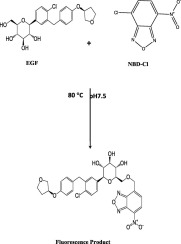
An efficient, accurate and sensitive spectrofluorimetric method was developed for analysis of empagliflozin (EGF) in pure form, dosage form and human plasma. The proposed procedure was based on formation of yellow fluorescent product between benzofurazan reagent and empagliflozin in slightly alkaline medium that is measured at 521 nm, when excitation at 455 nm. The present study was validated according to ICH guidelines and bioanalytical validated according to US-FDA guidance. The fluorescence intensity-concentration plot was linear over the range of 50-1000 ng ml-1 with limit of detection (LOD) and quantitation (LOQ) of 15.55 and 46.63 ng ml-1, respectively. The correlation (r) and determination (r2) coefficient was 0.9998 and 0.9997, respectively. Due to high sensitivity and selectivity of the proposed method, it is successfully used for analysis of empagliflozin in its dosage form and human plasma with good recoveries of 98.89% and 98.70%, respectively, without any interfering from matrix components. The corresponding regression equation, Y = 0.756X + 141.93, (r2 = 0.9994) for spiked plasma sample. Two level full factorial designs were used to study different experimental parameters that affect the reaction product and to get the optimum method conditions. The suggested method can be used in quality control lab as well as in pharmacokinetic studies of empagliflozin.
中文翻译:

使用两级全因子设计,可以对片剂和人体血浆中的依帕格列净进行新的荧光光谱分析。
开发了一种高效,准确和灵敏的荧光光谱法,用于分析纯形式,剂型和人血浆中的依帕列净(EGF)。拟议的程序是基于在弱碱性介质中苯并呋喃山试剂和依格列净之间形成黄色荧光产物的结果,当在455 nm激发时,在521 nm下测量。根据ICH指南验证了本研究,并根据US-FDA指南验证了生物分析。荧光强度-浓度图在50-1000 ng ml-1的范围内呈线性,检测限(LOD)和定量(LOQ)分别为15.55和46.63 ng ml-1。相关系数(r)和测定系数(r2)分别为0.9998和0.9997。由于所提出方法的高灵敏度和选择性,它已成功用于其剂型和人血浆中依帕列净的分析,回收率分别为98.89%和98.70%,而不会干扰基质组分。加标血浆样品的相应回归方程Y = 0.756X + 141.93(r2 = 0.9994)。两级全因子设计用于研究影响反应产物的不同实验参数并获得最佳方法条件。所建议的方法可用于质量控制实验室以及依帕列净的药代动力学研究。两级全因子设计用于研究影响反应产物的不同实验参数并获得最佳方法条件。所建议的方法可用于质量控制实验室以及依帕列净的药代动力学研究。两级全因子设计用于研究影响反应产物的不同实验参数并获得最佳方法条件。所建议的方法可用于质量控制实验室以及依帕列净的药代动力学研究。
更新日期:2020-03-27
中文翻译:

使用两级全因子设计,可以对片剂和人体血浆中的依帕格列净进行新的荧光光谱分析。
开发了一种高效,准确和灵敏的荧光光谱法,用于分析纯形式,剂型和人血浆中的依帕列净(EGF)。拟议的程序是基于在弱碱性介质中苯并呋喃山试剂和依格列净之间形成黄色荧光产物的结果,当在455 nm激发时,在521 nm下测量。根据ICH指南验证了本研究,并根据US-FDA指南验证了生物分析。荧光强度-浓度图在50-1000 ng ml-1的范围内呈线性,检测限(LOD)和定量(LOQ)分别为15.55和46.63 ng ml-1。相关系数(r)和测定系数(r2)分别为0.9998和0.9997。由于所提出方法的高灵敏度和选择性,它已成功用于其剂型和人血浆中依帕列净的分析,回收率分别为98.89%和98.70%,而不会干扰基质组分。加标血浆样品的相应回归方程Y = 0.756X + 141.93(r2 = 0.9994)。两级全因子设计用于研究影响反应产物的不同实验参数并获得最佳方法条件。所建议的方法可用于质量控制实验室以及依帕列净的药代动力学研究。两级全因子设计用于研究影响反应产物的不同实验参数并获得最佳方法条件。所建议的方法可用于质量控制实验室以及依帕列净的药代动力学研究。两级全因子设计用于研究影响反应产物的不同实验参数并获得最佳方法条件。所建议的方法可用于质量控制实验室以及依帕列净的药代动力学研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号