Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-03-26 , DOI: 10.1016/j.jmb.2020.03.020 M Moreno-Oñate 1 , A M Herrero-Ruiz 2 , M García-Dominguez 2 , F Cortés-Ledesma 3 , J F Ruiz 4
|
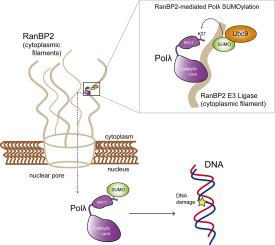
Cellular DNA is under constant attack by a wide variety of agents, both endogenous and exogenous. To counteract DNA damage, human cells have a large collection of DNA repair factors. Among them, DNA polymerase lambda (Polλ) stands out for its versatility, as it participates in different DNA repair and damage tolerance pathways in which gap-filling DNA synthesis is required. In this work, we show that human Polλ is conjugated with Small Ubiquitin-like MOdifier (SUMO) proteins both in vitro and in vivo, with Lys27 being the main target of this covalent modification. Polλ SUMOylation takes place in the nuclear pore complex and is mediated by the E3 ligase RanBP2. This post-translational modification promotes Polλ entry into the nucleus, which is required for its recruitment to DNA lesions and stimulated by DNA damage induction. Our work represents an advance in the knowledge of molecular pathways that regulate cellular localization of human Polλ, which are essential to be able to perform its functions during repair of nuclear DNA, and that might constitute an important point for the modulation of its activity in human cells.
中文翻译:

RanBP2介导的SUMOylation促进人类DNA聚合酶Lambda核定位和DNA修复。
细胞DNA受到各种内源性和外源性试剂的不断攻击。为了抵消DNA损伤,人类细胞具有大量的DNA修复因子。其中,DNA聚合酶lambda(Polλ)因其多功能性而出众,因为它参与需要填补缺口DNA合成的不同DNA修复和损伤耐受途径。在这项工作中,我们表明人Polλ在体外和体内均与小泛素样修饰物(SUMO)蛋白偶联。,其中Lys27是这种共价修饰的主要目标。PolλSUMOylation在核孔复合物中发生,并由E3连接酶RanBP2介导。这种翻译后修饰促进Polλ进入细胞核,这是其募集至DNA损伤所必需的,并受到DNA损伤诱导的刺激。我们的工作代表着对调节人类Polλ细胞定位的分子途径的了解的进步,这对于在修复核DNA期间能够执行其功能至关重要,并且可能构成调节其在人类体内活性的重要点。细胞。











































 京公网安备 11010802027423号
京公网安备 11010802027423号