当前位置:
X-MOL 学术
›
Free Radical Bio. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
αB-crystallin response to a pro-oxidant non-cytotoxic environment in murine cardiac cells: An "in vitro" and "in vivo" study.
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-03-26 , DOI: 10.1016/j.freeradbiomed.2020.03.013 Ambra Antonioni 1 , Ivan Dimauro 1 , Cristina Fantini 1 , Rosario Barone 2 , Filippo Macaluso 3 , Valentina Di Felice 4 , Daniela Caporossi 1
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-03-26 , DOI: 10.1016/j.freeradbiomed.2020.03.013 Ambra Antonioni 1 , Ivan Dimauro 1 , Cristina Fantini 1 , Rosario Barone 2 , Filippo Macaluso 3 , Valentina Di Felice 4 , Daniela Caporossi 1
Affiliation
|
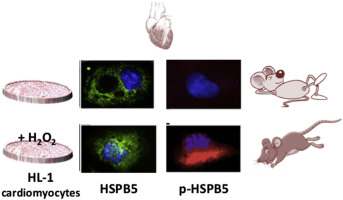
The αB-crystallin (HSPB5) protein is modulated in response to a wide variety of stressors generated by multiple physio-pathological conditions, sustained by reactive oxygen species (ROS) production. In cardiac muscle tissue, this protein regulates various cellular processes, such as protein degradation, apoptosis and the stabilization of cytoskeletal elements. In this work, we studied the role of HSPB5 expression, activation and localization in HL-1 murine cardiomyocytes exposed to pro-oxidant and non-cytotoxic H2O2 concentration, as well as in cardiac tissue isolated from mice following an acute, non-damaging endurance exercise. Our results demonstrated that HSPB5 is the most abundant HSP in both cardiac muscle tissue and HL-1 cells when compared to HSPB1 or HSPA1A (≈3-8 fold higher protein concentrations, p < 0.01). The acute exposure of cardiac muscle cells to sustainable level of H2O2 "in vitro" or to aerobic non-damaging exercise "in vivo" determined a fast and specific increase of HSPB5 phosphorylation (from 3 up to 25 fold increase, p < 0.01) correlated to an increase in lipid peroxidation (p < 0.05). In both experimental models, p-HSPB5 likely facilitated both the interaction with β-actin, desmin, and α-Filamin 1, the last one identified as new HSPB5 substrate in cardiac cells, as well as the sub-localization of HSPB5 within the same cellular compartment or the re-localization between compartments (i.e., nucleus and cytosol). Taken together, these data point out the role of "oxidative eustress" induced by physiological conditions in activating the molecular machinery devoted to cardiomyocytes' protection and candidate HSPB5 as a putative molecular mediator for the health benefits induced in cardiac tissue by exercise training.
中文翻译:

鼠心肌细胞中对氧化剂无细胞毒性环境的αB-晶状蛋白应答:“体外”和“体内”研究。
αB-晶状体蛋白(HSPB5)蛋白响应多种生理病理条件产生的应激源而受到调节,并由活性氧(ROS)产生维持。在心肌组织中,这种蛋白质调节各种细胞过程,例如蛋白质降解,细胞凋亡和细胞骨架元素的稳定。在这项工作中,我们研究了HSPB5表达,激活和定位在暴露于促氧化剂和无细胞毒性H2O2浓度的HL-1鼠心肌细胞中以及在急性,无破坏性耐力后从小鼠分离的心脏组织中的作用。行使。我们的结果表明,与HSPB1或HSPA1A相比,HSPB5在心肌组织和HL-1细胞中是最丰富的HSP(蛋白质浓度高约3-8倍,p <0.01)。心肌细胞“体外”或“无氧”无氧运动对心肌细胞可持续水平的急性暴露决定了HSPB5磷酸化的快速和特异性增加(从3倍增加到25倍,p <0.01)相关导致脂质过氧化作用增加(p <0.05)。在这两个实验模型中,p-HSPB5可能促进了与β-肌动蛋白,结蛋白和α-Filamin1的相互作用,后者被确定为心肌细胞中新的HSPB5底物,并且在同一细胞内HSPB5的亚定位细胞区室或区室之间的重新定位(即细胞核和胞质溶胶)。综上所述,这些数据指出了生理条件引起的“氧化性应激”在激活专门用于心肌细胞的分子机制中的作用。
更新日期:2020-03-27
中文翻译:

鼠心肌细胞中对氧化剂无细胞毒性环境的αB-晶状蛋白应答:“体外”和“体内”研究。
αB-晶状体蛋白(HSPB5)蛋白响应多种生理病理条件产生的应激源而受到调节,并由活性氧(ROS)产生维持。在心肌组织中,这种蛋白质调节各种细胞过程,例如蛋白质降解,细胞凋亡和细胞骨架元素的稳定。在这项工作中,我们研究了HSPB5表达,激活和定位在暴露于促氧化剂和无细胞毒性H2O2浓度的HL-1鼠心肌细胞中以及在急性,无破坏性耐力后从小鼠分离的心脏组织中的作用。行使。我们的结果表明,与HSPB1或HSPA1A相比,HSPB5在心肌组织和HL-1细胞中是最丰富的HSP(蛋白质浓度高约3-8倍,p <0.01)。心肌细胞“体外”或“无氧”无氧运动对心肌细胞可持续水平的急性暴露决定了HSPB5磷酸化的快速和特异性增加(从3倍增加到25倍,p <0.01)相关导致脂质过氧化作用增加(p <0.05)。在这两个实验模型中,p-HSPB5可能促进了与β-肌动蛋白,结蛋白和α-Filamin1的相互作用,后者被确定为心肌细胞中新的HSPB5底物,并且在同一细胞内HSPB5的亚定位细胞区室或区室之间的重新定位(即细胞核和胞质溶胶)。综上所述,这些数据指出了生理条件引起的“氧化性应激”在激活专门用于心肌细胞的分子机制中的作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号