Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and Function of the Bacterial Protein Toxin Phenomycin.
Structure ( IF 4.4 ) Pub Date : 2020-03-26 , DOI: 10.1016/j.str.2020.03.003 Bente K Hansen 1 , Camilla K Larsen 2 , Jakob T Nielsen 1 , Esben B Svenningsen 3 , Lan B Van 4 , Kristian M Jacobsen 3 , Morten Bjerring 1 , Rasmus K Flygaard 4 , Lasse B Jenner 5 , Lene N Nejsum 6 , Ditlev E Brodersen 4 , Frans A A Mulder 1 , Thomas Tørring 2 , Thomas B Poulsen 3
Structure ( IF 4.4 ) Pub Date : 2020-03-26 , DOI: 10.1016/j.str.2020.03.003 Bente K Hansen 1 , Camilla K Larsen 2 , Jakob T Nielsen 1 , Esben B Svenningsen 3 , Lan B Van 4 , Kristian M Jacobsen 3 , Morten Bjerring 1 , Rasmus K Flygaard 4 , Lasse B Jenner 5 , Lene N Nejsum 6 , Ditlev E Brodersen 4 , Frans A A Mulder 1 , Thomas Tørring 2 , Thomas B Poulsen 3
Affiliation
|
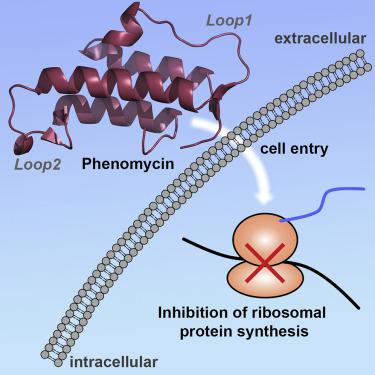
Phenomycin is a bacterial mini-protein of 89 amino acids discovered more than 50 years ago with toxicity in the nanomolar regime toward mammalian cells. The protein inhibits the function of the eukaryotic ribosome in cell-free systems and appears to target translation initiation. Several fundamental questions concerning the cellular activity of phenomycin, however, have remained unanswered. In this paper, we have used morphological profiling to show that direct inhibition of translation underlies the toxicity of phenomycin in cells. We have performed studies of the cellular uptake mechanism of phenomycin, showing that endosomal escape is the toxicity-limiting step, and we have solved a solution phase high-resolution structure of the protein using NMR spectroscopy. Through bioinformatic as well as functional comparisons between phenomycin and two homologs, we have identified a peptide segment, which constitutes one of two loops in the structure that is critical for the toxicity of phenomycin.
中文翻译:

细菌蛋白毒素苯菌素的结构和功能。
苯丙霉素是一种细菌的微型蛋白质,含50个氨基酸,在50年前被发现,在纳摩尔浓度的条件下对哺乳动物细胞具有毒性。该蛋白在无细胞系统中抑制真核核糖体的功能,并似乎靶向翻译起始。然而,关于苯霉素的细胞活性的几个基本问题仍未得到解答。在本文中,我们使用形态学分析来表明直接抑制翻译是苯霉素在细胞中的毒性基础。我们已经对苯霉素的细胞摄取机制进行了研究,表明内体逃逸是毒性限制步骤,并且我们已经使用NMR光谱法解决了蛋白质的溶液相高分辨率结构。
更新日期:2020-03-26
中文翻译:

细菌蛋白毒素苯菌素的结构和功能。
苯丙霉素是一种细菌的微型蛋白质,含50个氨基酸,在50年前被发现,在纳摩尔浓度的条件下对哺乳动物细胞具有毒性。该蛋白在无细胞系统中抑制真核核糖体的功能,并似乎靶向翻译起始。然而,关于苯霉素的细胞活性的几个基本问题仍未得到解答。在本文中,我们使用形态学分析来表明直接抑制翻译是苯霉素在细胞中的毒性基础。我们已经对苯霉素的细胞摄取机制进行了研究,表明内体逃逸是毒性限制步骤,并且我们已经使用NMR光谱法解决了蛋白质的溶液相高分辨率结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号