当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeting Orthosteric and Allosteric Pockets of Aromatase via Dual-Mode Novel Azole Inhibitors.
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-03-23 , DOI: 10.1021/acsmedchemlett.9b00591 Jessica Caciolla 1 , Angelo Spinello 2 , Silvia Martini 3 , Alessandra Bisi 1 , Nadia Zaffaroni 3 , Silvia Gobbi 1 , Alessandra Magistrato 2
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-03-23 , DOI: 10.1021/acsmedchemlett.9b00591 Jessica Caciolla 1 , Angelo Spinello 2 , Silvia Martini 3 , Alessandra Bisi 1 , Nadia Zaffaroni 3 , Silvia Gobbi 1 , Alessandra Magistrato 2
Affiliation
|
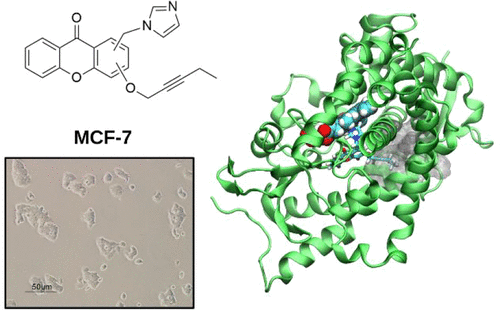
Breast cancer (BC) is the most diffused cancer type in women and the second leading cause of death among the female population. Effective strategies to fight estrogen responsive (ER+) BC, which represents 70% of all BC cases, rely on estrogen deprivation, via the inhibition of the aromatase enzyme, or the modulation of its cognate estrogen receptor. Current clinical therapies significantly increased patient survival time. Nevertheless, the onset of resistance in metastatic BC patients undergoing prolonged treatments is becoming a current clinical challenge, urgently demanding to devise innovative strategies. In this context, here we designed, synthesized, and performed in vitro inhibitory tests on the aromatase enzyme and distinct ER+/ER- BC cell line types of novel azole bridged xanthones. These compounds are active in the low μM range and behave as dual-mode inhibitors, targeting both the orthosteric and the allosteric sites of the enzyme placed along one access channel. Classical and quantum-classical molecular dynamics simulations of the new compounds, as compared with selected steroidal and nonsteroidal inhibitors, provide a rationale to the observed inhibitory potency and supply the guidelines to boost the activity of inhibitors able to exploit coordination to iron and occupation of the access channel to modulate estrogen production.
中文翻译:

通过双模式新型偶氮唑抑制剂靶向芳香化酶的正构和变构口袋。
乳腺癌(BC)是女性中最常见的癌症类型,并且是女性人群中第二大死亡原因。抵抗雌激素反应性(ER +)BC的有效策略(占所有BC病例的70%)依靠抑制芳香化酶或调节其相关雌激素受体来消除雌激素。当前的临床疗法显着增加了患者的生存时间。然而,在接受长期治疗的转移性BC患者中,耐药性的出现正成为当前的临床挑战,迫切需要制定创新的策略。在这种情况下,我们在这里设计,合成并进行了对芳香酶和新型唑桥杂吨酮的不同ER + / ER-BC细胞系类型的体外抑制试验。这些化合物在低μM范围内有活性,并充当双模式抑制剂,同时靶向沿着一个通道分布的酶的正构位和变构位。与选定的甾体和非甾体抑制剂相比,新化合物的经典和量子古典分子动力学模拟为观察到的抑制力提供了理论依据,并提供了指导准则,以增强能够利用铁的配位作用和对铁的占领的抑制剂的活性。调节雌激素产生的通道。
更新日期:2020-03-23
中文翻译:

通过双模式新型偶氮唑抑制剂靶向芳香化酶的正构和变构口袋。
乳腺癌(BC)是女性中最常见的癌症类型,并且是女性人群中第二大死亡原因。抵抗雌激素反应性(ER +)BC的有效策略(占所有BC病例的70%)依靠抑制芳香化酶或调节其相关雌激素受体来消除雌激素。当前的临床疗法显着增加了患者的生存时间。然而,在接受长期治疗的转移性BC患者中,耐药性的出现正成为当前的临床挑战,迫切需要制定创新的策略。在这种情况下,我们在这里设计,合成并进行了对芳香酶和新型唑桥杂吨酮的不同ER + / ER-BC细胞系类型的体外抑制试验。这些化合物在低μM范围内有活性,并充当双模式抑制剂,同时靶向沿着一个通道分布的酶的正构位和变构位。与选定的甾体和非甾体抑制剂相比,新化合物的经典和量子古典分子动力学模拟为观察到的抑制力提供了理论依据,并提供了指导准则,以增强能够利用铁的配位作用和对铁的占领的抑制剂的活性。调节雌激素产生的通道。









































 京公网安备 11010802027423号
京公网安备 11010802027423号